Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
June 2021
2: Rapid ATF4 Depletion Resets Synaptic Responsiveness after cLTP
3: Polygenic Risk Score for Alzheimer's Disease in Caribbean Hispanics
4: Vascular-Derived SPARC and SerpinE1 Regulate Interneuron Tangential Migration and Accelerate Functional Maturation of Human Stem Cell-Derived Interneurons
Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia
The Microtubule Cytoskeleton at the Synapse & The Synaptic Life of Microtubules
Optimizing Subjective Cognitive Decline to Detect Early Cognitive Dysfunction
The AD Tau Core Spontaneously Self-Assembles and Recruits Full-Length Tau to Filaments
Olfactory Impairment is Related to Tau Pathology and Neuroinflammation in Alzheimer's Disease
Pathogenic Role of Delta 2 Tubulin in Bortezomib-Induced Peripheral Neuropathy
Association of APOE E2 Genotype with Alzheimer's and Non-Alzheimer's Neurodegenerative Pathologies
APOE Ī4 and Resting-State Functional Connectivity in Racially/Ethnically Diverse Older Adults
Alzheimer-Related Cerebrovascular Disease in Down Syndrome
Endothelial Activation of Caspase-9 Promotes Neurovascular Injury in Retinal Vein Occlusion
Tau is not Necessary for Amyloid-Beta-Induced Synaptic and Memory Impairments
IL-27: An Endogenous Constitutive Repressor of Human Monocytes
"Everything Hurts!" Distress in Semantic Variant Primary Progressive Aphasia
Down Syndrome: Distribution of Brain Amyloid in Mild Cognitive Impairment
Cortical Thickness and its Associations with Age, Total Cognition and Education Across the Adult Lifespan
Structural Brain Changes in Pre-Clinical FTD MAPT Mutation Carriers
Phospholipase D1 Ablation Disrupts Mouse Longitudinal Hippocampal Axis Organization and Functioning
Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains
Profilin 1 Delivery Tunes Cytoskeletal Dynamics Toward CNS Axon Regeneration
Human Herpesvirus 6 Detection in Alzheimer's Disease Cases and Controls Across Multiple Cohorts
APOE4 is Associated with Differential Regional Vulnerability to Bioenergetic Deficits in Aged APOE Mice
Microglial Activation, but not Tau Pathology, is Independently Associated with Amyloid Positivity and Memory Impairment
CRISPR/Cas9 Editing of APP C-Terminus Attenuates β-Cleavage and Promotes α-Cleavage
Activity-Dependent Nucleation of Dynamic Microtubules at Presynaptic Boutons Controls Neurotransmission
Role of Tau Protein in Remodeling of Circadian Neuronal Circuits and Sleep
Pum2 Shapes the Transcriptome in Developing Axons through Retention of Target mRNAs in the Cell Body
Promotion of Axon Growth by the Secreted End of a Transcription Factor
Increased Diameters of the Internal Cerebral Veins and the Basal Veins of Rosenthal Are Associated with White Matter Hyperintensity Volume
Neuroinflammation in Frontotemporal Lobar Degeneration Revealed by 11CāPBR28 PET
Live Imaging of ESCRT Proteins in Microfluidically Isolated Hippocampal Axons
Alzheimer's Association International Conference (AAIC 2019)
Association of Variants in PINX1 and TREM2 With Late-Onset Alzheimer Disease
Brain Biomarkers and Cognition Across Adulthood
Brain Arterial Dilatation and the Risk of Alzheimer's Disease
FDG-PET Patterns Associated with Underlying Pathology in Corticobasal Syndrome
Effect of Aerobic Exercise on Cognition in Younger Adults: A Randomized Clinical Trial
Exercise-linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimerās Models
A Tau Homeostasis Signature Is Linked with the Cellular and Regional Vulnerability of Excitatory Neurons to Tau Pathology
Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain
Semantic Network Function Captured by Word Frequency in Nondemented APOE Īµ4 Carriers
First Place: NSUN2 is Dysregulated in Alzheimer's Disease
Homeostatic Plasticity Scales Dendritic Spine Volumes and Changes the Threshold and Specificity of Hebbian Plasticity
An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
Clinical Experience with Cerebrospinal Fluid AĪ²42, Total and Phosphorylated Tau in the Evaluation of 1,016 Individuals for Suspected Dementia
A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research
Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
Preparation of Tau Oligomers After the Protein Extraction from Bacteria and Brain Cortices
Medical Retirement from Sport after Concussions: A Practical Guide for a Difficult Discussion
Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
A Transcriptomic Atlas of Aged Human Microglia
Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-terminal Fragments and Unique Lipid Signatures
Marked Mild Cognitive Deficits in Humanized Mouse Model of Alzheimer's-Type Tau Pathology
 |  | |
| Joshua D. Cho, PhD Student | Ismael Santa-Maria Perez, PhD |
Dysregulation of tau proteostasis can arise from a number of sources such as: splice site and/or missense mutations, changes in overall expression or isoform composition, various post-translational modifications, and epigenetic regulators. These alterations in tau proteostasis can lead to the abnormal misfolding of tau, resulting in the eventual seeding and spread of pathological tau species that contribute to a number of neurodegenerative diseases, collectively known as tauopathies, which includes Alzheimerās Disease. Therefore, elucidating the mechanisms of tau protein regulation is imperative to unraveling the underlying mechanisms of these neurodegenerative diseases. To this aim the htau mouse model was generated, which recapitulates many of the tau associated histopathological features of Alzheimerās Disease. However, behavioral analyses of this model have yielded inconsistent results in the past. This lack of consistency led our laboratory to collaborate with Dr. Mu Yang (director of the Institute of Genomic Medicine Mouse NeuroBehavior Core) on a study recently published in Frontiers in Behavioral Neuroscience. In this study, we sought to further characterize and verify the presence/robustness of cognitive phenotypes in this mouse model before using it for further investigation of disease mechanisms or to screen for novel treatment strategies.
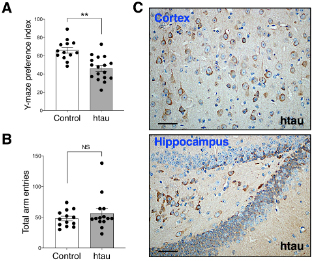
Figure: Htau mice show impaired spatial novelty preference in Y Maze. Ā (A)Ā Preference index was significantly reduced in 16-18 months htau mice in comparison to controls.Ā (B)Ā Total arm entries are similar in htau and controls during the Y maze test, ruling out motor confounds. **p < 0.01 vs. control (control,Ā NĀ = 13; htau,Ā NĀ = 17).Ā (C)Representative immunohistochemistry images from the cortex and hippocampus showing accumulation of tau pathology (stained with phospho-specific tau AT8 antibody) in old htau mice. Brain areas shown are involved in the task performance. Scale bars: 120 Ī¼m. All values are expressed as means Ā± SEM.
This research, led by first author Joshua D. Cho, a PhD student in the lab, shows behavioral data collected from a carefully curated battery of learning and memory tests. We found a significant short-term spatial memory deficit in the Novel Arm Y-Maze test among 16ā18-month-old htau mice; representing a novel finding in this tauopathy mouse model. However, we did not find salient impairments in long-term learning and memory tests (such as Morris Water Maze) that were previously reported in these mice. We attempted to understand the discrepancies in the literature by highlighting the necessity of scrutinizing key procedural differences across studies and concluded that the reported cognitive deficits in the htau model depend on task difficulty and other procedural details, such as task repetition or the absence/presence of strong or adverse incentive stimuli. Thus, while the htau mouse remains a valuable animal model for replicating late onset AD-like human tau pathology, its cognitive deficits are modest under standard testing conditions. Our findings serve as a key reminder of the importance of properly characterizing and standardizing the behavioral assessment on mouse models of neurodegenerative disease before evaluating treatment efficacies or embarking on research aimed to understand the mechanisms of the disease.
Ismael Santa-Maria Perez, PhD
Assistant Professor of Pathology and Cell Biology
is2395@cumc.columbia.edu
Rapid ATF4 Depletion Resets Synaptic Responsiveness after cLTP
 |  | ||
| Fatou Amar, PhD | Carlo Corona, PhD | ||
 |  | ||
| Michael Shelanski, MD, PhD | Lloyd A. Greene, PhD |
Our ability to form new memories depends on changes in synaptic activity within the brain. Among the key components of this plasticity is the process of long-term potentiation (LTP) in which activation of a chemical synapse evokes a persistent increase in responsiveness to subsequent synaptic stimulation. While LTP can be long-lasting, it also requires a mechanism to reset to baseline so that the affected synapses can participate in new memory formation and do not reach potentially destructive levels of activity. Our group has long been interested in the role of the transcription factor ATF4 in the brain and has reported that its presence is required for normal synaptic plasticity and activity. ATF4 is a particularly attractive player in such roles because unlike most transcription factors, it is present at synapses and can be retrogradely transported to cell nuclei where it directs gene regulation. ATF4 also has the unusual property that its expression levels can be rapidly regulated by alteration of its synthesis and degradation.
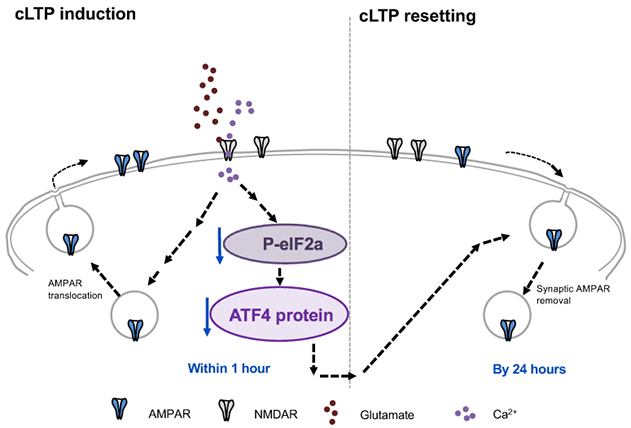
Figure 9. Model for role of ATF4 regulation in resetting of excitatory synapse density and potentiation in response to cLTP. Glutamate-induced chemical LTP leads to a rapid downregulation of ATF4 protein levels via decreased phosphorylation of eIF2a and consequent reduction in ATF4 mRNA translation. The resulting ATF4 protein decrease is required for active resetting of synaptic AMPARs density, thereby returning synaptic responses to baseline levels to prevent saturation and to make synapses more responsive to future stimuli.
In the present study by Amar et al., we asked whether ATF4 could play a role in resetting of LTP. To approach this question, we studied mature cultures of brain hippocampal neurons in which LTP was chemically induced by brief exposure to the neurotransmitter glutamate. In this system, LTP was induced within minutes, persisted for at least 1 hour and was no longer present by 24 hours, as recently reported in eNeuro. Protein measurement and immunostaining revealed that LTP induction was accompanied by a rapid, significant decrease in ATF4 expression. This loss was first detected in dendrites where synapses are present and then, in cell bodies where ATF4 enters the nucleus to direct transcription of proteins relevant to synaptic activity. Like LTP, the reduction in ATF4 levels was transient and returned to baseline by 24 hours following LTP induction. Additional experiments established that the LTP-dependent decline in ATF4 levels was due to decreased local synthesis and was dependent on activation of the NMDA subtype of glutamate receptors. These observations raised the key question of whether the observed fall in ATF4 levels caused by LTP was in turn responsible for the subsequent resetting of LTP to baseline. To answer this, we used viral infection to constitutively express ATF4 in neurons so that its levels did not appreciably diminish after LTP induction. While maintaining ATF4 levels in this way did not affect the initial establishment of LTP, it interfered with LTP resetting so that in contrast to control neurons, neurons with elevated ATF4 expression retained LTP for at least 24 hours. These findings led us to conclude that ATF4 plays a required role in regulating LTP duration. This appears to occur via a mechanism in which synaptic activity that induces LTP reduces ATF4 expression, and that this reduction in turn leads to LTP extinction, retuning neurons to a state in which they can again participate in a new round of plasticity associated with learning and memory.
While our study probed the role of ATF4 in normal brain function, the findings also have potential relevance to neurodegenerative disorders such as Alzheimerās and Parkinsonās diseases. Multiple reports have documented increases in ATF4 in neurons of patients with these maladies. Just as in our investigation in which experimental elevation of ATF4 blocked the resetting of LTP in normal neurons, the high levels of ATF4 that are present in neurons of AD or PD patients could interfere with LTP extinction, and by this means disrupt learning and memory. Moreover, overly prolonged LTP driven by ATF4 over-expression in such cases could lead to hyperactivity that could result in neuronal deterioration. These considerations support the idea that development of means to reduce ATF4 levels or activity could be ameliorative in certain neurodegenerative diseases.
Lloyd A. Greene, PhD
Professor of Pathology and Cell Biology
lag3@cumc.columbia.edu
Polygenic Risk Score for Alzheimer's Disease in Caribbean Hispanics
 |  |  | ||
| Sariya Sanjeev, MSc | Richard Mayeux, MD, MSc | Giuseppe Tosto, MD, PhD |
Polygenic risk score (PRS) is a popular method that generates a disease risk score at individual level by combining sparse information across the genome. So far most of the PRS have been developed for individuals with European ancestry, thus, PRS applicability and performance remain unknown in under-represented ethnicities such as Caribbean Hispanics.
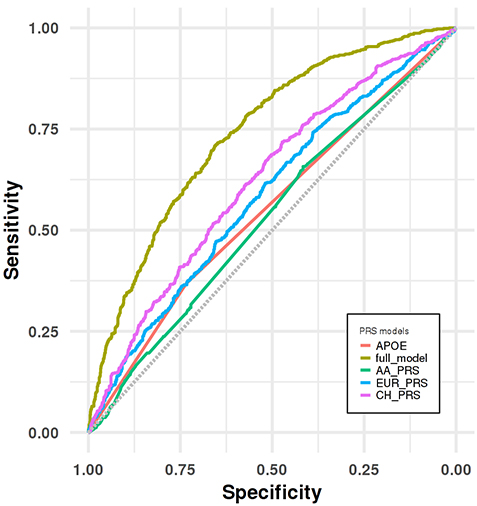
Figure 3. Receiver operating characteristic (ROC) curve analyses for distinguishing LOAD group from cognitive healthy control group in the CH validation dataset.
In the present study by Sariya et al., we generated the first PRS in Caribbean Hispanics for the Late-onset Alzheimerās Disease (LOAD) using the WHICAP, EFIGA and 10/66 Puerto Rico projects. As recently reported in the Annals of Neurology, we showed a good prediction accuracy (Area under the curve= 74%) when PRS is combined with other well-established risk factors such as age, sex and APOE, comparable to PRS generated in larger studies of European ancestry. We also validated this newly developed PRS by showing the association between higher PRS scores and neuropathological features in an independent Caribbean Hispanics autopsy cohort. Additionally, we replicated this association in an independent, case-control, Caribbean Hispanic cohort (part of the NIA Alzheimerās Disease Center study) and showed that PRS significantly predicts conversion from healthy status to LOAD in longitudinal analyses. Finally, we demonstrated that our PRS was superior to alternative PRS generated using large European and African American GWAS, proving that diversity in genetic studies is critical for effective PRS in LOAD and other diseases.
Giuseppe Tosto, MD, PhD
Assistant Professor of Neurology (in the Taub Institute and the Sergievsky Center)
gt2260@cumc.columbia.edu
 |  | |
| Edmund Au, PhD | Gunnar Hargus, MD, PhD |
Cortical interneurons establish inhibitory microcircuits throughout the neocortex and their dysfunction has been linked to various human disorders. Developmentally, interneurons migrate from a distal progenitor domain in order to populate the neocortex - a process that occurs at a slower rate in humans than in mice. Indeed, since all aspects of maturation are slower in human versus mouse neurons, methods must be developed to accelerate human neuronal maturation for the purpose of disease modeling. In this study, published in eLife and led by Dr. Edmund Au (Pathology & Cell Biology), we conducted a cross-species analysis and found that interneuron migration is regulated by vascularization of the interneuron progenitor domain embryonically. This effort was aided by samples obtained from our Neuropathology lab, as well as collaborations with Drs. Joseph Gogos (Psychiatry), Peter Canoll (Pathology & Cell Biology) and Dritan Agalliu (Neurology). Two endothelial cell-derived paracrine factors, SPARC and SerpinE1, that enhance interneuron migration in mice were identified. The group applied these factors to human stem cell-derived cortical interneurons and found that pre-treatment prior to transplantation into neonatal mouse cortex enhanced human interneuron migration. Surprisingly, it also increased the morphological complexity of human interneurons and their electrophysiological characteristics were also more mature in the treated group. These findings indicate a critical role for CNS vasculature in regulating interneuron developmental maturation in both mice and humans and introduces a method to accelerate the functional maturation rate of human stem cell-derived neurons.
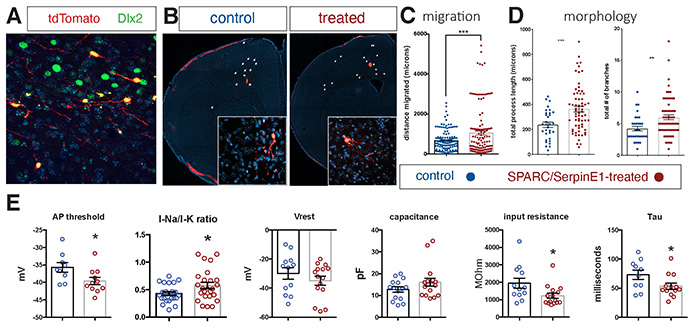 Figure. Human stem cell-derived interneurons xenografted into host mouse cortex are more migratory and more morphologically-complex and more functionally mature with SPARC and SerpinE1 pre-treatment. (A) Immunohistochemistry for Dlx2 (green), Dapi (blue) and tdTomato+ hSC-interneurons (red) near injection site 1-month post-transplant. (B) Representative images of coronal sections of host mouse cortex 1 month post-transplant of control untreated and SPARC/SerpinE1 pre-treated hSC-interneurons (asterisk denotes transplant site). Arrowheads show tdTomato+ hSC-interneurons migrating away from transplant site. (C) Quantification of migratory distance of hSC-interneurons from site of injection 1-month post transplantation. (D) Quantification of hSC-interneuron morphology by average process length (left) and total number of neurite branches (right). (E) Whole cell recordings of hSC-interneurons 2-months post-transplant. AP threshold (mV) (p=0.0166), sodium-to-potassium current ratio (p=0.0338), resting membrane potential (p=0.12), capacitance (p=0.067), input resistance (p=0.0155), Tau (p=0.0239). Paired t-test, * p < 0.05; **p < 0.01; *** p < 0.001. |
Human stem cell-derived neurons (hSC-neurons) represent an attractive model for studying diseases of the nervous system. However, before this can be achieved, effective strategies must be developed to allow hSC-neurons to functionally mature in a consistent and timely fashion. This is especially true of neurodegenerative disorders such as Alzheimerās disease (AD) or AD-related diseases (ADRD) in which mature neuronal function is a prerequisite to fully reveal disease phenotypes. Two prominent obstacles impeding hSC-neuron functional maturation are: 1) the need for an appropriate environment in which to mature, and 2) a species-specific slow rate of human neuronal maturation. This study demonstrates that both of these obstacles (accelerated maturation and transplantation into mouse cortex) can be addressed synergistically to study mature human neurons in a more naturalistic setting. Relatedly, together with my co-investigators in the Taub Institute ā Drs. Vilas Menon and Andrew Sproul ā we are analyzing hSC-neurons with the AD/ADRD-related MAPTN279K or APPV717I mutations after transplantation into the mouse brain. Using a combination of immunohistochemistry and single nucleus RNA sequencing of grafts, we aim to identify novel disease pathways and potential therapeutic targets for the benefit of patients.
Gunnar Hargus, MD, PhD
Assistant Professor of Pathology and Cell Biology
gh2374@cumc.columbia.edu
Edmund Au, PhD (Corresponding Author)
Assistant Professor of Pathology and Cell Biology
ea2515@cumc.columbia.edu

