Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
May 2021
2: Socioeconomic and Psychosocial Mechanisms Underlying Racial/Ethnic Disparities in Cognition Among Older Adults
3: Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia
 Natura Myeku, PhD
Natura Myeku, PhDAccumulation of pathological tau in synapses has been identified as an early event in Alzheimer’s disease (AD) and correlates with cognitive decline in patients with AD. In normal conditions, tau is cytosolic axonal protein that stabilizes microtubules, and its level in synapses is low. Under disease conditions, however, tau accumulates in postsynaptic compartments and presynaptic terminals, due to missorting within neurons, transsynaptic transfer between neurons, or a failure of clearance pathways. Using subcellular fractionation of brain tissue from rTg4510 tau transgenic mice with tauopathy and human postmortem brain tissue from patients with AD, a new study by Dr. Natura Myeku, first author Ari Schaler, and colleagues from Taub separated synapses into pre- and postsynaptic compartments to show that, in AD brains, synaptic tau accumulates predominantly in the postsynaptic compartments, making these structures vulnerable to tau toxicity. As recently reported in Science Translational Medicine and featured on CUIMC Newsroom, these postsynaptic compartments contained not only a higher amount of tau, but the tau contained was more pathogenic, acting as a seed to recruit naïve tau into aggregates.

Figure: A, B) In Alzheimer’s disease, seed-competent synaptic tau accumulates predominately in the post-synaptic compartment contributing to synaptic dyshomeostasis. C, D) Receptor-mediated clearance of tau in the post-synaptic compartment by the local proteasomes could be a new approach to protect synapses from toxic tau.
Previous research by Dr. Myeku and colleagues has shown that tau aggregation in mice can be attenuated by enhancing the clearance of tau via proteasome machinery. Thus, to combat the accumulation of tau and proteasome impairment in the postsynaptic compartments of rTg4510 mouse brain, the investigators targeted a receptor situated on the surface of postsynaptic compartments, stimulating the pituitary adenylate cyclase–activating polypeptide (PACAP) type 1 receptor (PAC1R). Mice with early-stage tau pathology treated with PACAP exhibited reduced postsynaptic tau, reduced hyperphosphorylated synaptic tau, and overall attenuated tauopathy. In addition, these rTg4510 transgenic mice demonstrated improved cognitive performance on two behavioral tests. These results suggest that activating PAC1R could prevent accumulation of aggregate-prone tau and indicate a potential therapeutic approach for AD and other tauopathies.
Natura Myeku, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
nm2631@cumc.columbia.edu
 |  | |
| Laura Zahodne, PhD | Adam M. Brickman, PhD |
Non-Hispanic Black and Hispanic older adults experience disproportionate burden of Alzheimer’s disease and related dementias (ADRD) compared with non-Hispanic Whites. Substantial research indicates that these racial/ethnic differences in ADRD risk and prevalence reflect health inequalities that are avoidable, unnecessary, and unjust. Socioeconomic factors, such as educational attainment and income, clearly contribute to ADRD inequalities, but these variables fail to fully explain group differences. An emerging literature highlights other modifiable psychosocial factors that contribute to racial/ethnic differences in ADRD risk above and beyond socioeconomic factors, such as racial discrimination.
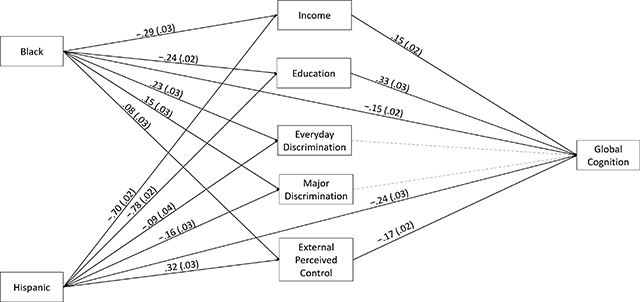 Figure 1.
Mediation Model. Significant Paths are Shown as Black Lines, With Corresponding Standardized Parameter Estimates and Standard Errors.Nonsignificant Paths are Depicted With Gray, Dotted Lines. For Simplicity, Direct Effects, Covariates (i.e., Age and Sex/Gender) and Covariances Between Mediators are Not Shown.
|
Recently, a battery of psychosocial measures was added to the long-running Washington Heights-Inwood Columbia Aging Project in order to more fully identify factors that create and sustain racial/ethnic inequalities in ADRD. As reported in Neuropsychology, Drs. Laura Zahodne, Adam Brickman, and colleagues from Taub, Neurology, and the University of Michigan used mediation analyses to quantify the proportion of Black-White and Hispanic-White disparities in late-life cognitive performance explained by various social determinants of health. They found that socioeconomic factors (i.e., educational attainment, income) explained approximately 50% of cognitive disparities. An additional 5-8% of disparities were explained by external locus of control, as measured by individuals’ self-reported perceptions of outside constraints that limit their ability to achieve important life outcomes. While there were also racial/ethnic differences on a commonly-used measure of everyday discrimination such that Black older adults reported more frequent discrimination than Hispanics or Whites, everyday discrimination was not uniquely associated with cognitive performance.
These results suggest that external locus of control may be an under-recognized contributor to ADRD inequalities. As an individual-level indicator of chronic institutional and interpersonal marginalization, external locus of control may represent not only an upstream determinant of known drivers of health disparities (e.g., economic opportunity) but also an independent driver of disparities that could operate via stress and/or other biopsychosocial pathways.
Laura B. Zahodne, PhD
Adjunct Associate Research Scientist (in Neurology and the Taub Institute)
Assistant Professor of Psychology, University of Michigan
lzahodne@umich.edu
Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia
 |  | |
| Megan S. Barker, PhD | Stephanie Cosentino, PhD |
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disease that primarily affects personality and behavior. Executive dysfunction, that is deficits in higher-order cognitive skills such as judgment and reasoning, is typically considered the cognitive hallmark of bvFTD. However, recent research has shown that episodic memory deficits, which are more prototypical of Alzheimer’s disease than bvFTD, may also be present in up to 50% of bvFTD patients. Furthermore, there is some evidence that episodic memory deficits may be more common in carriers of a MAPT genetic mutation, which is a specific type of tau mutation that causes bvFTD, compared with other bvFTD-causing genetic mutations (C9orf72, GRN). Whether episodic memory deficits are detectable at the earliest symptomatic (“prodromal”) stage of disease, and whether there are differences between genetic mutation carrier groups at this prodromal disease stage, remains largely unexamined in the literature.
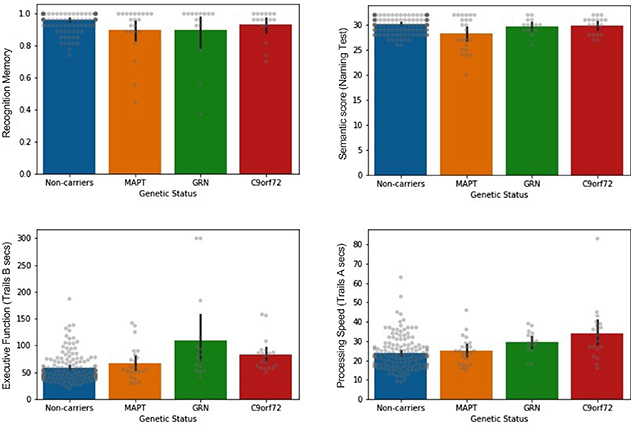 Figure.
Bar plots showing mean performance on memory and other cognitive tests, compared across genetic carrier (MAPT, GRN, C9orf72) and non-carrier control groups. Descriptive only (uncorrected). Error bars represent 95% confidence intervals. Raw data depicted in gray.
|
In a study recently published in the journal Cortex, Drs. Stephanie Cosentino, Ted Huey, and colleagues, including postdoctoral research scientist and lead author Dr. Megan Barker, investigated patterns of episodic memory and cognitive impairment in prodromal bvFTD. Specifically, they sought to understand the extent to which difficulty recognizing words from a previously learned list reflected impairment in memory storage per se, versus impairment in semantic (language) or executive functioning, which are the cognitive domains more classically associated with bvFTD. Leveraging data from 57 carriers of a pathogenic variant of the MAPT (N = 23), GRN (N = 15), or C9orf72 (N = 19) genes, who will go on to develop bvFTD in the future but were exhibiting very mild symptoms at the time of the study, the authors reported that episodic memory dysfunction is detectable in prodromal bvFTD. That is, the patient group was less successful at recognizing words from the target list than controls. Of the genetic groups, this deficit was most reliably observed in MAPT and GRN mutation carriers. However, in MAPT carriers, the episodic memory weakness appeared to reflect a genuine memory difficulty, as well as a semantic (language) disruption, while, in contrast, the memory problems in GRN carriers seemed to be underpinned by executive dysfunction. Episodic memory was most preserved in the C9orf72 group, but processing speed was mildly slowed in this group as compared to controls. Overall, these results suggest that distinct cognitive weaknesses are evident in carriers of the three disease-causing genes during the prodromal disease stage, and may differentially contribute to poor performance on episodic memory tests.
Megan S. Barker, PhD
Postdoctoral Research Scientist (in the Taub Institute)
msb2228@cumc.columbia.edu
Stephanie Cosentino, PhD
Associate Professor of Neuropsychology (in Neurology, the Sergievsky Center, and the Taub Institute)
sc2460@cumc.columbia.edu

