Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
June 2020
2: » IL-27: An Endogenous Constitutive Repressor of Human Monocytes
3: » Subgingival Microbiome and Clinical Periodontal Status in an Elderly Cohort: The WHICAP Ancillary Study of Oral Health
Tau is not Necessary for Amyloid-Beta-Induced Synaptic and Memory Impairments

Ottavio Arancio, MD, PhD
The prevailing “amyloid cascade” hypothesis in Alzheimer’s disease (AD) research posits that amyloid-ß (Aß) and tau proteins are placed in series with Aβ upstream of tau, in a sort of trigger-bullet mechanism. This hierarchical profile in the chain of events leading to memory loss in AD is used as an explanation for the failures of many clinical trials, mostly targeting Aß. Two types of strategies are currently being implemented to overcome this obstacle. In one line of research, anti-Aß therapies are being administered prior to the overt disease manifestation. In the other, various aspects of tau pathology, including tau post-translational modifications, or tau levels, or tau aggregation status are being targeted. This hypothesis has more recently been challenged, however, by studies suggesting that Aß and tau act in parallel instead of being in series. If, in fact, the two proteins act in parallel, both anti-Aß and anti-tau therapies alone are doomed to fail. Solving this conundrum has been a central focus of research in the laboratory of Dr. Ottavio Arancio.
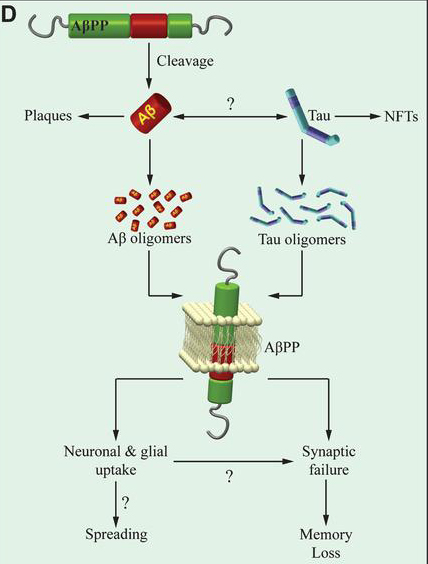
In the current manuscript, Dr. Arancio and colleagues, including former postdoc and first author Daniela Puzzo (University of Catania) report breakthrough findings to further inform this debate. Now published online in The Journal of Clinical Investigation, they demonstrate that neither exogenous oAß nor oTau need endogenous mouse tau to negatively impact the late phase of CA3-CA1 LTP and long-term hippocampal memory. Moreover, they find that tau suppression not only fails to reduce amyloid load in a mouse model of Aß deposition, but prompts the unraveling of a defect in basal neurotransmission in the model. These findings suggest that, at least for certain electrophysiological, behavioral, and histopathological aspects, Aß and tau act in parallel, and not in series as the amyloid cascade hypothesis predicts. Most importantly, Dr. Arancio and colleagues note, “our findings suggest that therapies targeting simultaneously Aß and tau might effectively improve LTP and memory. This might be achieved by combining anti-Aß and anti-tau therapeutics, or more likely, given that the physiological functions of these proteins might render these therapeutics not clinically viable, targeting substrates downstream of both peptides through either personalized medicine approaches or drugs acting on second messenger systems shared by the two proteins and relevant to synaptic plasticity and memory.”
Ottavio Arancio, MD, PhD
Professor of Pathology and Cell Biology (in the Taub Institute)
oa1@cumc.columbia.edu
IL-27: An Endogenous Constitutive Repressor of Human Monocytes
 |  | |
| Elizabeth M. Bradshaw, PhD | Wassim Elyaman, PhD |
The pleiotropic cytokine, Interleukin (IL)-27, has long been studied for its abilities to modulate T cell phenotypes in health and disease. As monocytes are known to be a major producer of IL-27, a new study by Drs. Elizabeth Bradshaw, Wassim Elyaman, and colleagues, including first author Dr. Michael Frangieh (Brigham and Women’s Hospital/Harvard), sought to examine the effect of endogenously produced IL-27 on human monocytes. As recently reported in the journal Clinical Immunology, they found that the addition of an anti-IL-27 neutralizing antibody increased the production of pro-inflammatory cytokines ex vivo with no additional stimuli needed (Figure 1). Neutralizing monocyte-derived IL-27 leads to monocytes that more readily polarize T cells to the Th17 phenotype, but have no effect on Th1 polarization. These findings suggest that IL-27 functions as an endogenous constitutive repressor, limiting pro-inflammatory cytokine production by keeping monocytes in a steady state. It also modifies the ability of monocytes to induce differentiation of CD4+ T cells to the Th17 phenotype. The locus that contains the IL27 gene has been linked to susceptibility for type 1 diabetes (T1D). Interestingly, ex vivo monocytes from subjects with T1D produce more IL-27.
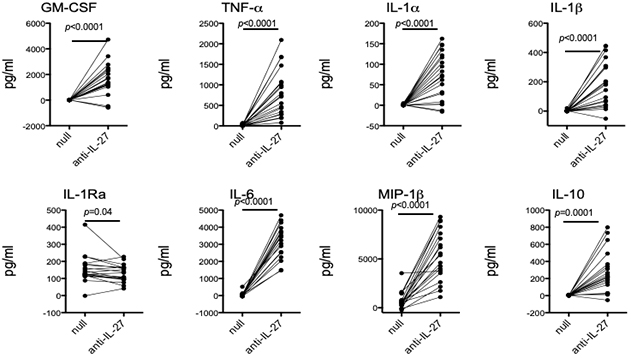 Figure 1: Neutralization of endogenous IL-27 leads to increased cytokine production. Negatively isolated monocytes from healthy subjects were incubated with an IL-27 neutralizing antibody for 40 hours. Supernatants were collected and cytokine production was measured using the Luminex platform. Each individual is represented as a dot.
|
As it becomes clearer that the immune system, both innate and adaptive, plays an important role in neurodegenerative diseases, such as Alzheimer and Parkinson disease, understanding pleiotropic cytokines, such as IL-27, in health and disease becomes a priority for neurodegenerative disease research. Unlike autoimmune diseases, where it is known that the immune system is inappropriately activated, in neurodegenerative diseases it is less clear if the immune system needs to be dampened down to reduce inflammation or ramped up to overcome immunosenescence brought on with age and disease. The receptor for IL-27 is widely expressed on immune cells, but also on non-immune cells including endothelial cells and astrocytes. Therefore, it is likely to have far reaching influence beyond modulating infiltrating T cell phenotypes. IL-27 itself is produced in the CNS by microglia, in addition to its peripheral production by circulating monocytes. Serum levels of IL-27 were found to be reduced in patients with Parkinson disease compared to healthy age matched individuals. Yet, among patients, levels were highest in individuals with severe disease compared to those with mild or moderate symptoms, as recently reported by Kouchaki et al. Understanding this master regulator could open up new avenues to understanding the role of the immune system in neurodegeneration.
Elizabeth M. Bradshaw, PhD
Adler Assistant Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Institute for Genomic Medicine)
emb2280@cumc.columbia.edu
Wassim Elyaman, PhD
Assistant Professor of Neurological Sciences (in Neurology, the Taub Institute and the Institute for Genomic Medicine)
we2152@cumc.columbia.edu
 James M. Noble, MD, MS
James M. Noble, MD, MSOver the past 20 years, the Washington Heights-Inwood-Hamilton Heights Columbia Aging Project (WHICAP) has serially assessed approximately 6,000 participants over the age of 65 years with respect to medical, social, and health behavior histories, general medical exams, and neuropsychological testing. The WHICAP Ancilliary Study of Oral Health, led by Dr. James Noble, is an NIH-funded cohort study involving a subset of 1,130 individuals from the WHICAP cohort, aimed at studying the relationship between periodontitis—a marker of systemic inflammation—and cognitive decline.
In the current study, now published online in the Journal of Periodontology, Dr. Noble and colleagues, including first author Dr. Panos Papapanou (Director of the Division of Periodontics, Columbia College of Dental Medicine) and Anne-Catrin Uhlemann (Director of the Columbia Microbiome Core Facility), present data on the subgingival microbiome of the dentate participants of the ancillary study, and of the association of metrics of bacterial relative abundance and diversity with clinical periodontal status. The investigators obtained and analyzed plaque samples by means of next generation sequencing to carry out a comprehensive identification of the prevalent bacterial taxa as well as to calculate relative abundance and diversity metrics in different states of periodontal health and disease. They classified the clinical periodontal status (see figure) using both a four-level ordinal scale that is widely used in epidemiological studies (the CDC/AAP classification) and a continuous measure of periodontitis extent and severity based on the percentage of teeth per participant with pockets ≥4 mm deep.
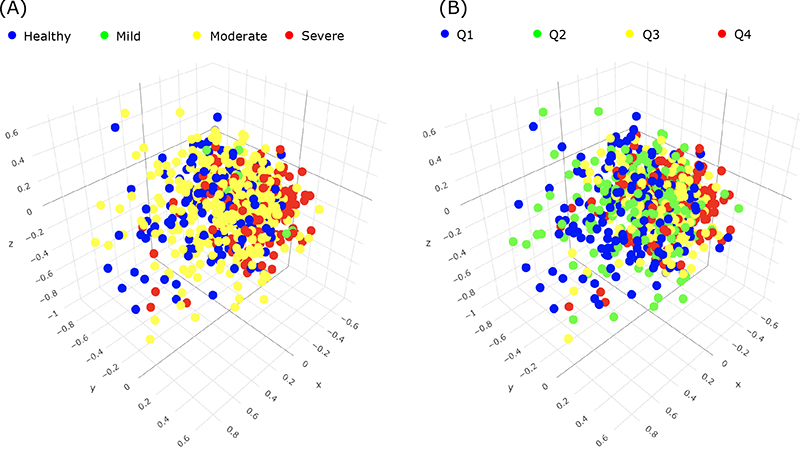 Figure 3A: Periodontal disease groups relative to indices of abundance and diversity of periodontal microbes.
|
Findings from Papapanou et al. indicate that 1) the most abundant and/or differentially enriched taxa that emerged among the distinct periodontal phenotypes in this cohort of elderly individuals were generally similar to those described in the literature for younger age groups; and 2) subgingival microbial diversity increased in parallel with the severity and extent of periodontitis.
This research is part of a broader, ongoing study exploring cross-sectional and longitudinal indicators of cognitive change relative to these periodontal markers of disease. Preliminary studies leading to this current project were supported by the Department’s T32 neuroepidemiology fellowship training grant and Taub pilot research support.
James M. Noble, MD, MS
Associate Professor of Neurology (in the Taub Institute and the Sergievsky Center) at the Columbia University Medical Center
jn2054@cumc.columbia.edu

