Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
September 2019
» 2: Synaptic and Memory Dysfunction Induced by Tau Oligomers is Rescued by up-regulation of the Nitric Oxide Cascade
3: » Medicaid Contributes Substantial Costs to Dementia Care in an Ethnically Diverse Community Population
 |  | |
| Jose Gutierrez-Contreras, MD, MPH | Adam M. Brickman, PhD |
White matter hyperintensities (WMH) on T2-weighted magnetic resonance (MR) imaging are typical in older adults and have been linked to several poor health outcomes, including cognitive impairment and Alzheimer’s disease (AD). The presence and severity of WMH have traditionally been attributed to occlusive arteriopathy, but recent evidence also implicates deep medullary venule collagenosis and associated vasogenic edema. Historically, postmortem analyses have been the sole way to analyze cerebral veins, but susceptibility-weighted imaging (SWI)—a new, non-invasive technique to enhance contrast in MR imaging—can now used to examine cortical veins in vivo.
A new study by the laboratory of Dr. Adam Brickman, along with HHMI student and lead author Alexander Houck (University of Tennessee) and Columbia Neurologist Dr. Jose Gutierrez, used SWI to visualize and measure the diameters of large draining veins in the brain to test the hypothesis that cerebral basal vein dilation is related to WMH volume. As recently published in the American Journal of Neuroradiology, they found that increased diameters of the internal cerebral veins and the basal veins of Rosenthal were associated with greater WMH volume. The strongest association for the internal cerebral veins was in the parietal lobe, the brain region where past studies have most consistently identified a correlation between WMH and AD. The strongest association for the basal veins of Rosenthal was in the frontal lobe.
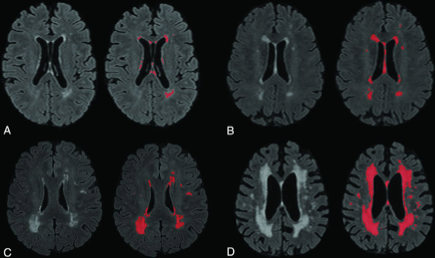
Figure 1: Four sample images of WMH on T2 FLAIR, labeled for quantification using an intensity threshold with our software. A, Minimal WMHs. B, Mild WMHs. C, Moderate WMHs. D, Severe WMHs.
Overall, their study provides evidence of the relationship between WMHs and both the internal cerebral veins and the basal veins of Rosenthal. According to the authors, "it is unclear whether this association is merely correlative or if there is a causation between these pathologies. There are many avenues that can expand on this research in the future." The Brickman lab is in the process of developing methods for perivascular space quantification, which could be used to determine whether these spaces relate to cerebral venous diameter. The authors further suggest additional topics of analyses and techniques worthy of pursuit, concluding that "with the aging population, the clinical implications are clear: Understanding the etiology of this relationship might lead to therapeutic and preventative techniques to combat vascular disease and its downstream effects."
Adam M. Brickman, PhD
Associate Professor of Neuropsychology
amb2139@cumc.columbia.edu

Ottavio Arancio, MD, PhD
Soluble aggregates of oligomeric forms of tau protein (oTau) have been associated with impairment of synaptic plasticity and memory in Alzheimer’s disease (AD). However, despite the strong correlation between oTau and AD pathology, the molecular mechanisms by which tau protein induces synaptic dysfunction and memory impairment remain unknown.
There is a broad consensus that cyclic adenosine monophosphate (cAMP) responsive element binding (CREB) protein plays a crucial role in memory consolidation. CREB is at the crossroads of several molecular pathways and mechanisms that have been proposed as potential therapeutic targets against AD, including the nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) dependent protein kinases (PKG)/CREB pathway, which is particularly attractive because drugs boosting it, especially phosphodiesterase 5 (PDE5) inhibitors, are widely used for the therapy of erectile dysfunction and pulmonary hypertension, and it is therefore plausible that their administration is compatible with therapeutic usage.
NO, a gaseous molecule produced by the NO-synthase enzyme is involved in various steps of brain physiology, from development to synaptic plasticity and memory. Intriguingly, proteomic and metabolomic studies have revealed disrupted NO homeostasis in AD [33]. Moreover, up-regulation of the NO cascade through drugs acting on its various molecular components has provided favorable results in studies aimed at finding strategies to counteract the damage of synaptic plasticity and memory by oligomers of Aβ, another toxic protein in AD. Given that oTau share a common molecular mechanism with Aβ oligomers when they impair memory and long-term potentiation (LTP) in mice, a new study by the laboratory of Dr. Ottavio Arancio, including lead author Erica Acquarone, along with Dr. Jole Fiorito from the New York Institute of Technology, used a combination of biochemical, electrophysiological and behavioral techniques to investigate whether the oTau-induced damage of synaptic function and memory can be rescued via up-regulation of the NO cascade.
As recently published in Molecular Neurodegeneration, their findings provide a novel view on how oTau affects synaptic plasticity and memory, pointing at the NO cascade as a second messenger pathway that can be exploited to counteract tau-induced damage of synaptic plasticity and memory (Figure 9), and offering a new window of therapeutic opportunities against AD and other neurodegenerative diseases characterized by an increase in oTau.
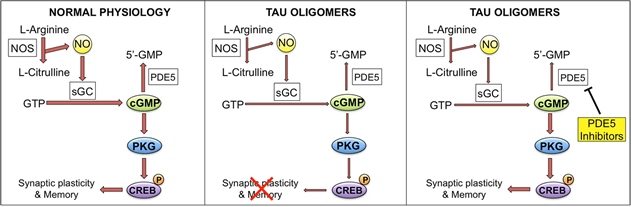 Figure 9: oTau effect on the NO signaling cascade. Left panel shows the cascade under physiological conditions. NO is produced by the enzyme nitric oxide synthase (NOS) that converts L-arginine into L-citrulline. NO activates soluble guanilyl cyclase (sGC), which produces cyclic guanosine monosphosphate (cGMP) from guanosine triphosphate (GTP). cGMP is degraded into 5'-GMP by phosphodiesterase 5 (PDE5). The increase of cGMP levels activates cGMP-dependent protein kinase (PKG), which induces phosphorylation of cAMP-responsive element binding (CREB) and enhancement of synaptic plasticity and memory. In the presence of oTau (central panel), phosphorylation of CREB is reduced as the signaling cascade is down regulated (shown by narrower arrows), leading to an impairment of synaptic plasticity and memory processes. Treatment with PDE5 inhibitors (right panel) rescues the levels of cGMP leading to increased CREB phosphorylation and normal synaptic plasticity and memory.
|
Ottavio Arancio, MD, PhD
Professor of Pathology and Cell Biology (in the Taub Institute)
oa1@cumc.columbia.edu

Yaakov Stern, PhD
In 2019, the Alzheimer’s Association (AA) reported national average annual Medicaid payment per person for individuals with dementia ($8,565) to be 23 times greater than for those without dementia ($365). The vast majority of studies that do not include Medicaid spending may substantially underestimate increased total payer costs related to dementia care. A new study by Dr. Yaakov Stern and colleagues from Neurology and Taub, along with first author Dr. Carolyn Zhu (Mount Sinai), sought to identify a more comprehensive perspective on the expenditures associated with dementia by examining data with both Medicare and Medicaid claims from the multiethnic Washington Heights-Inwood Columbia Aging Project (WHICAP).
Now published in The Journals of Gerontology, results show that in participants who had full Medicaid coverage, average annual Medicaid expenditures were substantially higher for those with dementia than those without ($50,270 vs. $21,966, p<0.001), but Medicare expenditures did not differ by dementia status ($8,458 vs. $9,324, p=0.19). In participants who did not have any Medicaid coverage, average annual Medicare expenditures were substantially higher for those with dementia than those without dementia ($12,408 vs. $8,113, p=0.02). In adjusted models, dementia was associated with a $6,278 increase in annual Medicaid spending per person after controlling for other characteristics. This work provides important insight regarding the cost of dementia in a diverse, extremely vulnerable population by highlighting cost differences due to dementia related to Medicaid.
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center, and in the Taub Institute)
ys11@cumc.columbia.edu

