Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
March 2020
 |  |  | ||
| Estela Area Gomez, PhD | Karen Duff, PhD | Tal Nuriel, PhD |
While much of the research on the association of the apolipoprotein E4 (APOE4) allele and Alzheimer’s disease (AD) risk has focused on the ability of the apoE4 protein to increase the aggregation and decrease the clearance of Aβ, there is also an abundance of data showing that APOE4 negatively impacts many additional processes in the brain, including bioenergetics. In order to gain a more comprehensive understanding of the diverse effects that APOE4 expression has on the brain, the laboratories of Dr. Tal Nuriel, Dr. Estela Area Gomez, and Dr. Karen Duff teamed up to perform a series of investigations in aged APOE mice.
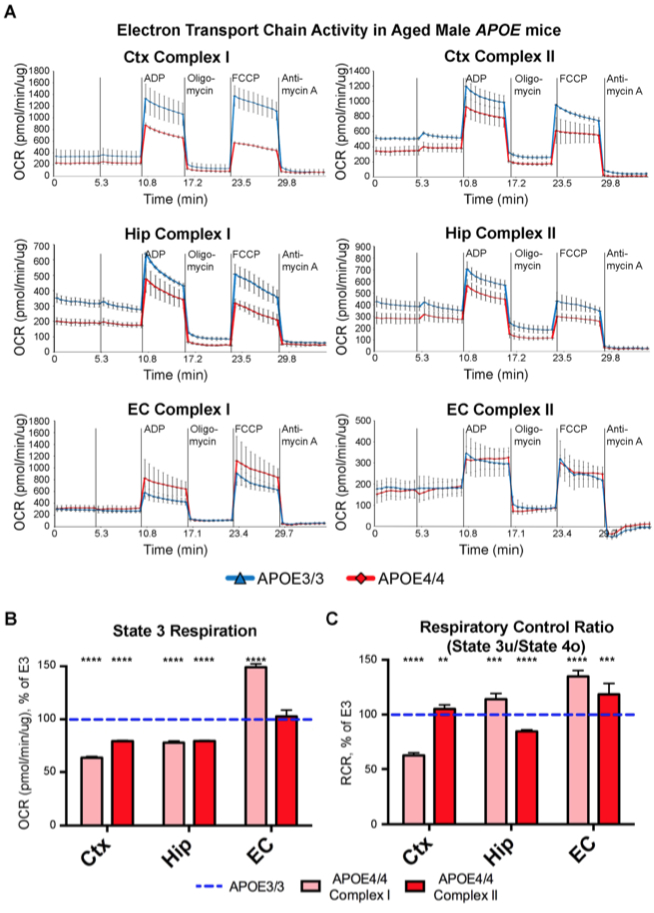 Figure 3: Seahorse analysis reveals decreased mitochondrial respiration in the cortex and hippocampus, but not in the EC, of aged male APOE4 mice. Seahorse analysis was performed in order to analyze the effects of differential APOE isoform expression on mitochondrial respiration in mitochondria that were isolated from the cortex (Ctx), hippocampus (Hip) and EC of 20-month-old APOE mice (2 APOE4/4 males, tissues pooled vs. 2 APOE3/3 males, tissues pooled). (A) The oxygen consumption rate (OCR) from each region shows decreased mitochondrial respiration in the Ctx and Hip, but not the EC of the aged APOE4/4 mice. (B,C) Bar graphs showing the average OCR from (B) State 3 and for (C) the Respiration Control Ratio (RCR; state 3 u/state 4o) in each region of the APOE4/4 mice, as a percentage of the APOE3/3 OCR from the equivalent tissues. The dotted blue line represents the normalized levels in the APOE3/3 tissues. (**denotes p < 0.01; ***denotes p < 0.001; ****denotes p < 0.0001).
Figure 3: Seahorse analysis reveals decreased mitochondrial respiration in the cortex and hippocampus, but not in the EC, of aged male APOE4 mice. Seahorse analysis was performed in order to analyze the effects of differential APOE isoform expression on mitochondrial respiration in mitochondria that were isolated from the cortex (Ctx), hippocampus (Hip) and EC of 20-month-old APOE mice (2 APOE4/4 males, tissues pooled vs. 2 APOE3/3 males, tissues pooled). (A) The oxygen consumption rate (OCR) from each region shows decreased mitochondrial respiration in the Ctx and Hip, but not the EC of the aged APOE4/4 mice. (B,C) Bar graphs showing the average OCR from (B) State 3 and for (C) the Respiration Control Ratio (RCR; state 3 u/state 4o) in each region of the APOE4/4 mice, as a percentage of the APOE3/3 OCR from the equivalent tissues. The dotted blue line represents the normalized levels in the APOE3/3 tissues. (**denotes p < 0.01; ***denotes p < 0.001; ****denotes p < 0.0001).
As reported this month in Scientific Reports, they found that while aged APOE4 mice possess bioenergetic deficits in the hippocampus and cortex, the entorhinal cortex (EC) of these mice appears to possess unique counterbalancing mechanisms that allows it to resist these APOE4-associated decreases in mitochondrial respiration. Specifically, they observed increased coupling of oxygen consumption with ATP production in the EC of aged APOE4 mice, which appears to be modulated by an upregulation of multiple mitochondrial nutrient shuttling systems. Finally, the authors hypothesize that this increased coupling efficiency may give way to increased ROS production, which may in turn play a causative role in the early AD pathology observed in the EC during disease pathogenesis.
Estela Area Gomez, PhD
Assistant Professor in the Department of Neurology
eag2118@cumc.columbia.edu
Tal Nuriel, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute) at the CUMC
tn2283@cumc.columbia.edu
Profilin 1 Delivery Tunes Cytoskeletal Dynamics Toward CNS Axon Regeneration
 Francesca Bartolini, PhD
Francesca Bartolini, PhDAdult vertebrate central nervous system (CNS) axons are unable to regenerate. This failure results from the highly inhibitory extrinsic environment at the injury site, and the intrinsic inability of CNS axons to reactivate actin and microtubule remodeling to form a competent growth cone from a dystrophic growth-incompetent retraction bulb. In the present study, an international team of investigators, including Taub faculty member Dr. Francesca Bartolini, use in vivo models with high and low regenerative capacities, followed by gain- and loss-of-function analysis, to identify the actin-binding protein profilin 1 (Pfn1) as a critical regulator of actin and microtubule dynamics that supports axon regrowth and regeneration in mouse models of nerve injury. Peripheral nerve injury leads to increased Pfn1 activity in growth cones, where it promotes cytoskeletal dynamics that enhance axon regeneration. Loss of Pfn1, on the other hand, impairs axon growth in both peripheral and spinal cord injury as well as reduces axonal and dendritic growth in cultured hippocampal neurons. As published in The Journal of Clinical Investigation, the authors examine the mechanism of cytoskeletal regulation and demonstrate that in the growth cone, Pfn1 interferes both with microtubule growth and invasion into filopodia through cooperation with formins, a family of actin nucleators and modulators of microtubule stability. Importantly, systemic delivery of Pfn1 in mice promotes axon regeneration of injured sciatic nerves, and of transected ascending spinal cord tracts (see the accompanying Figure), demonstrating the potential of Pfn1-targeting therapies to enhance nerve regrowth and functional recovery after injury.
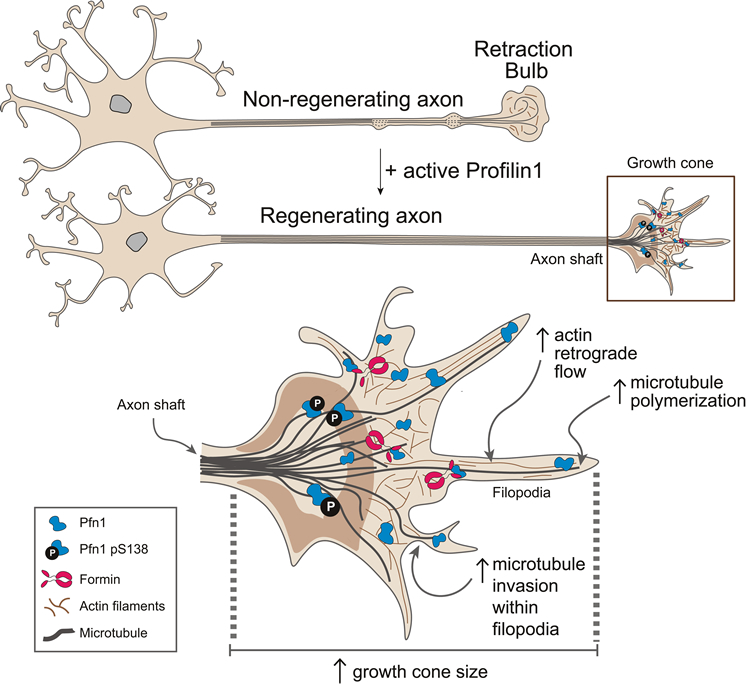
Mutations in Pfn1 have been associated with neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), further underscoring the role of Pfn1 in maintaining a healthy neuronal architecture. The findings in this paper demonstrate that modulation of Pfn1 levels and activity is instrumental to achieve a regenerative phenotype and suggest that AAV-mediated delivery of active Pfn1 and/or identification of in vivo modulators of Pfn1 activity, should be considered for the treatment of the injured nervous system as well as neurodegenerative disease.
Francesca Bartolini, PhD
Assistant Professor of Pathology and Cell Biology at the CUMC
fb2131@cumc.columbia.edu
Human Herpesvirus 6 Detection in Alzheimer's Disease Cases and Controls Across Multiple Cohorts

Philip L. De Jager, MD, PhD
Several recent studies have renewed the debate concerning the role of herpesviruses, and human herpesvirus 6 (HHV-6) in particular, in Alzheimer’s disease (AD). Thus, Dr. Philip L. De Jager and members of his lab collaborated with colleagues from the National Institute of Neurological Disorders and Stroke (NINDS) to screen for HHV-6 detection across three independent AD brain repositories.
First, using the Broad Institute PathSeq tool, RNA sequencing datasets from the Mount Sinai Brain Bank and the Religious Orders Study/Memory and Aging Project (ROSMAP) were screened for pathogens against taxon references from over 25,000 microbes, including 118 human viruses. Next, using a highly sensitive and precise digital droplet polymerase chain reaction (ddPCR) platform, DNA samples from 711 AD and control brains obtained from ROSMAP and the Johns Hopkins Brain Resource Center were probed for PCR reactivity to HHV-6A and/or HHV-6B.
As published in Neuron, these direct methods of viral detection did not support an association between HHV-6 and AD but also did not rule it out. According to the authors, “if viruses (and HHV-6 in particular) do play a role in AD pathogenesis, then the agents may no longer be present in a form that can be PCR amplified or sufficiently expressed. Rather, these virus(es) may be associated with an earlier “triggering” event or be present at copy numbers below the limit of laboratory detection.”
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu

