Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
January 2019
2: » Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain
 |
| Karen Duff, PhD |
As detailed in a recent Nature Neuroscience review by Drs. Karen Duff, Hongjun (Harry) Fu, and John Hardy (Institute of Neurology, London), understanding the molecular origins of selective neuronal vulnerability is of fundamental importance for all neurodegenerative diseases. Previous research by Dr. Duff and others has found that the distribution of neurons vulnerable to tauopathy follows a sequential pattern that suggests that cell populations in different regions of the brain are selectively at risk. More specifically, the morphology and location of cells within the entorhinal cortex (EC) and hippocampus that accumulate tau and degenerate in the earliest stages of Alzheimer's disease (AD) suggest that excitatory (EX) neurons are preferentially impacted. However, the exact molecular determinants underlying the selective vulnerability of EX neurons to tau pathology have not been established.
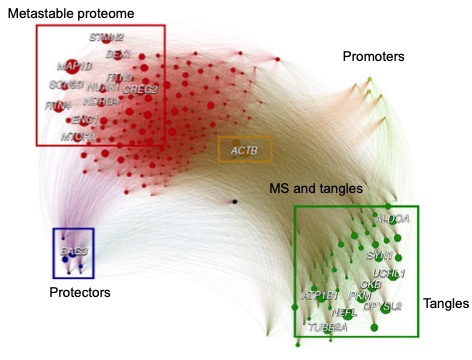
Figure 1: Co-expression network analysis of the subproteomes relevant to tau homeostasis.
Prompted by recent observations that age-related stress and dysfunction of protein homeostasis are observable in vulnerable neurons in aging and age-related neurodegenerative diseases, Drs. Duff and Fu (now at Ohio State), along with Dr. Michele Vendruscolo from the University of Cambridge, sought to explore these determinants by employing four complementary approaches that allowed the investigators to probe individual cells in mouse and human brain, as recently reported in Nature Neuroscience. First, using a series of cell-type-specific markers on AD patient brains and a mouse model of tauopathy previously generated by Dr. Duff’s laboratory, they showed that tau co-localizes predominantly with EX, compared to inhibitory (IN) neuron markers, not only in the EC but also in areas affected later in the disease, such as the neocortex. Second, using single-nucleus RNA-seq datasets from normal donors, they identified a substantial difference between EX and IN neurons in genes involved in a branch of the protein homeostasis system that modulates the aggregation and clearance of tau. Third, using the weighted gene co-expression network analysis, they identified that BCL2-associated athanogene 3 (BAG3), a facilitator of autophagy, was identified as a hub, or master regulator, gene in the co-expression network relevant to tau homeostasis. Lastly, they confirmed that BAG3 is differentially expressed in human EX and IN neurons in non-AD and AD brains and that it impacts tau accumulation in primary neurons.
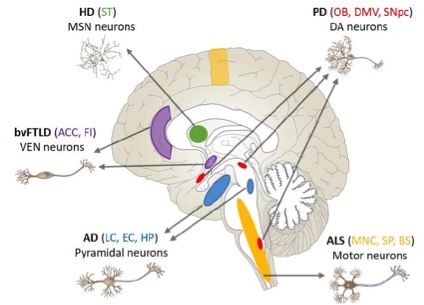
Figure 2: Regional vulnerability in neurodegenerative diseases.
Taken together, these results support the conclusion that tau homeostasis contributes to the selective regional vulnerability of EX neurons to tau pathology and cell loss that defines AD, and suggest that dysregulation of specific branches of the protein homeostasis system plays an important role in the initiation and spread of tau pathology in AD and the primary tauopathies. A lay summary of these findings is currently available on CUIMC Newsroom.
Karen Duff, PhD
Deputy Director, Taub Institute
Professor of Pathology and Cell Biology (in Psychiatry and in the Taub Institute)
ked2115@columbia.edu
 |  | |
| Eleanna Varangis, PhD | Yaakov Stern, PhD | |
 |  | |
| Christian Habeck, PhD | Qolamreza (Ray) Razlighi, PhD |
Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain
Recent studies of the effect of aging on the brain have uncovered numerous ways in which brain structure and function change over the course of adulthood. In many cases, these age-related brain changes are linked to co-occurring cognitive changes, suggesting that different aspects of brain health may predict cognitive outcomes in the context of healthy aging. Recent studies in this field have turned to functional connectivity methodology to probe this relationship between brain function and cognitive outcomes, and have found support for the idea that inefficient patterns of functional connectivity between networks in the brain while at rest may underlie declining cognitive performance with aging. However, little is known about how network interactions during a cognitive task may be related to performance on that task, how different tasks may differentially affect the connectivity among networks in the brain, or how aging might play a role in these between-network interactions during a task.
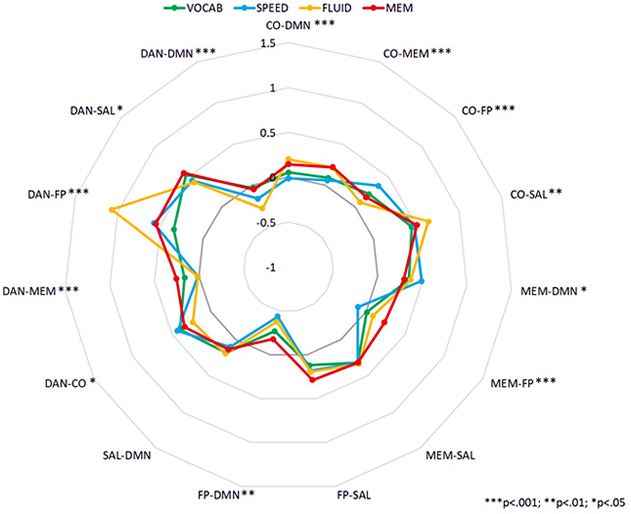
Figure: All ages: connection x domain.
Together with Taub investigators Drs. Yaakov Stern, Christian Habeck, and Qolamreza (Ray) Razlighi, Dr. Eleanna Varangis examined connectivity among six cognitive networks during the performance of eleven different fMRI tasks stemming from four primary cognitive domains: Vocabulary, Processing Speed, Fluid Reasoning, and Episodic Memory. Participants were 142 healthy adults between the ages of 20-80. As published in the Journal of Cognitive Neuroscience, their study demonstrated that both participant age and cognitive task domain have independent effects on patterns of between-network connectivity. The results also showed that the strength of some of the connections between networks was related to task performance, with several of the relationships between task performance and connectivity varying by participant age.
These results provide support for the idea that older adults show different patterns of connectivity between cognitive networks while performing a cognitive task, and that some aspects of this connectivity may be related to cognitive performance. Further, this study is also the first to demonstrate differences in functional connectivity patterns during a cognitive task as a function of task domain, suggesting that the brain can dynamically reorganize itself to support a variety of cognitive functions throughout adulthood.
Eleanna Varangis, PhD
Postdoctoral Research Scientist (in the Gertrude H. Sergievsky Center)
ev2171@cumc.columbia.edu
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center, and in the Taub Institute)
ys11@cumc.columbia.edu

