Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
Best Poster Presentations
Taub Institute Retreat November 2018
» #1 Homeostatic Plasticity Scales Dendritic Spine Volumes and Changes the Threshold and Specificity of Hebbian Plasticity
» #2 An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
» #1 A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research
» #2 Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
» #2 Preparation of Tau Oligomers After the Protein Extraction from Bacteria and Brain Cortices
» #2 Medical Retirement from Sport after Concussions: A Practical Guide for a Difficult Discussion
» #1 Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
» #2 White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
» #1 ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
» #2 Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
» #3 A Transcriptomic Atlas of Aged Human Microglia
» #1 Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-terminal Fragments and Unique Lipid Signatures
» #1 Neuronal Hyperactivity Due to Loss of Inhibitory Tone in APOE4 Mice Lacking Alzheimer's Disease-Like Pathology
and
» The Endosomal–Lysosomal Pathway Is Dysregulated by APOE4 Expression in Vivo
» First Place: A CSF Proteomic Screen Links Retromer to Alzheimer's Pathogenic Pathways and Suggests Endosomal-Trafficking Biomarkers
» First Place: Microglia Identity in the Aged and AD Human Brain
» #1 Intra-Axonal Synthesis of SNAP25 is Required for the Formation of Presynaptic Terminals
» #1 Stabilization of Dynamic Microtubules by mDia1 Drives Tau-dependent Aβ1-42 Synaptotoxicity
» #2 LTP and Memory Impairment Caused by Extracellular Aβ and Tau Oligomers is APP-Dependent
» #2 Neuropathologic Features of TOMM40 '523 Variant on Late-Life Cognitive Decline
» #2 An Approach to Studying the Neural Correlates of Reserve
» #1 Brain Atrophy Can Introduce Age-Related Differences in BOLD Response
» #2 Age-Related Biomarkers in LLFS Families With Exceptional Cognitive Abilities
» #2 Polygenic Risk Scores in Familial Alzheimer Diseases
» #1 Local Synthesis of Dynein Cofactors Matches Retrograde Transport to Acutely Changing Demands
» #3 Relation of Dysglycemia to Structural Brain Changes in a Multiethnic Elderly Cohort
NSUN2 is Dysregulated in Alzheimer’s Disease
 |
| Pictured (from left to right): Dr. Jennifer J. Manly, Yoon A. Kim, and Dr. Inbal Israely |
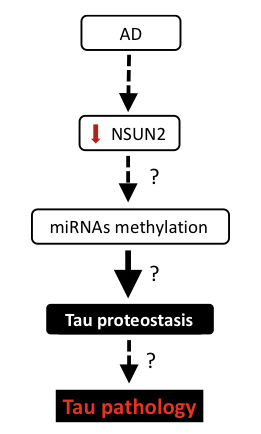 |
| Figure: Investigating the role of NSUN2 in Alzheimer's disease. |
Yoon A. Kim1,2 & Ismael Santa-Maria1,2
1Department of Pathology & Cell Biology, Columbia University Medical Center, New York, NY.
2Taub Institute for Research on Alzheimer's Disease and the Aging Brain.
Accumulation of extracellular amyloid beta (Aβ) deposits, intracellular neuronal tangles composed of hyperphosphorylated tau and synaptic loss are the main hallmarks of Alzheimer’s disease (AD), the most prevalent form of dementia. Currently, the molecular basis of AD is unclear. However, several studies support that altered microRNA (miRNA) expression and/or function plays an important role in AD pathogenesis. However, the mechanisms governing how miRNAs are regulated in the brain are poorly understood. RNA methylation is a prevalent posttranscriptional modification that regulates accuracy of translation initiation, RNA stability, biogenesis and processing of RNA. Historically, it has been shown that methylation occurs on transfer RNA, ribosomal RNA and messenger RNA. Recently, methylation of miRNAs has also been found. However, methyltransferases involved in this process are still under investigation. NSUN2 is one the few known brain-enriched methyltransferases in higher eukaryotes that is able to mediate methylation of miRNAs. Moreover, the loss of NSUN2 in Drosophila and mouse models causes memory and learning deficits, indicating a potential role of NSUN2 in cognitive function. Furthermore, in humans, mutations in the NSUN2 gene cause intellectual disability. Interestingly, the role of microRNA methylation in AD pathogenesis has not been reported. Here, our data supports dysregulation of NSUN2 in post-mortem brain tissue from AD patients when compared to healthy controls. In addition, we found that oligomeric Aβ induces both dysregulation of NSUN2 and changes in tau proteostasis in primary neuronal cultures. Furthermore, bioinformatic analysis shows predicted methylation sites in miRNAs that have been implicated in AD, supporting a possible link between NSUN2 dysfunction, microRNAs and AD pathogenesis.
Yoon A. Kim
Student, Graduate Program in Pathobiology and Molecular Medicine
Columbia University Irving Medical Center
yk2477@cumc.columbia.edu
High-throughput Disease Modeling to Uncover Shared and Unique Characteristics Among Neurodegenerative Diseases
 |
| Pictured (from left to right): Dr. Inbal Israely, Samuel Resnick, and Dr. Jennifer J. Manly |
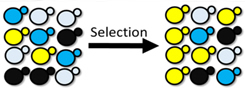 |
| Figure: DNA-barcoded libraries allow us to multiplex high-throughput screening by studying multiple disease models in the same screen. This application towards neurodegenerative diseases gives us the ability to rapidly and efficiently identify novel phenotypic modifiers for a wide range of genes implicated in neurodegeneration. |
Samuel Resnick 1,2,3 & Alejandro Chavez 1,2
1 Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University
2 Department of Pathology and Cell Biology, Columbia University
3 Columbia University Vagelos College of Physicians and Surgeons
The ability to assay multiple cellular models of disease in parallel against a wide variety of experimental conditions in lieu of traditional one-at-a-time approaches would greatly increase the scope of known cellular responses in disease. Here, we develop a scalable, inexpensive, and multiplexed approach to identify genetic modifiers of aggregation-prone proteins implicated in a variety of neurodegenerative diseases during a single experiment. Using DNA-barcoded yeast, we screen upwards of 4000 potential interactions between aggregation-prone proteins and molecular chaperones and subsequently identify numerous interactions that rescue aggregation-prone protein mediated cellular toxicity. These hits are comprised of previously reported chaperone-aggregate interactions in addition to novel interactions that will be followed up in mammalian models of disease.
Samuel Resnick
Student, Graduate School of Arts and Sciences
Columbia University Irving Medical Center
sjr2179@cumc.columbia.edu

