Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
January 2018
2: » An Inflammation-Related Nutrient Pattern is Associated with Both Brain and Cognitive Measures in a Multiethnic Elderly Population
 |  | |
| Gilbert Di Paolo, PhD | André Miguel Miranda |
Defects in the endolysosomal pathway are increasingly associated with neurodegenerative disorders, including Alzheimer's disease (AD) and Parkinson's disease (PD). These defects, which result in many cases from mutations in genes encoding key regulators of endolysosomal function, are believed to compromise the health of neurons by causing accumulation of toxic proteins or lipids. A key disease-associated protein is amyloid precursor protein (APP), whose aberrant metabolism has been historically and causally-linked to AD pathology via accumulation of amyloid-beta (Aβ) peptide in senile plaques. More recent studies have emphasized a pathogenic role for other APP fragments, such as APP C-terminal fragments beta (APP-CTFβ) (i.e., a product of β-secretase) in AD. However, the impact of endolysosomal dysfunction on APP processing in AD or other disorders is far from being understood.
Now published in Nature Communications, one recent report from the lab of Gil Di Paolo, by first author André Miranda, highlights the fact that neuronal endolysosomal dysfunction impacts APP-CTFs much more profoundly than Aβ both in vitro and in vivo. Teaming up with Dr. Scott Small, the authors observed that endolysosomal dysfunction results in robust release of APP-CTFs that are associated with extracellular vesicles derived from secretory endosomes, namely exosomes. These vesicles carry also unique lipid signatures, including lipids derived from the endolysosomal system. A key message of this study is that neurons have the capacity to secrete undigested proteins and lipids via exosomes when their lysosomes are compromised, allowing them to alleviate their lysosomal storage disorder.
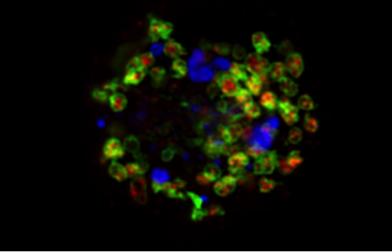
Figure 1: Bis (monoacylglycero) phosphate (BMP, shown in red), also known as lysobisphosphatidic acid, is an atypical phospholipid predominantly found in internal vesicles of late endosomes/lysosomes (LAMP-1, shown in green). Miranda et al. report that disruption of neuronal PI3P/Vps34 signaling leads to endolysosomal membrane damage labeled in part with p62 (shown in blue) and aberrant release of undigested lysosomal material in APP-CTF- and BMP-positive exosomes.
Miranda et al. followed up on published observations from the Di Paolo lab showing that phosphatidylinositol-3-phosphate (PI3P), a phospholipid synthesized by the lipid kinase Vps34, is deficient in AD patients' brain and mouse models thereof (Morel et al. Nature Commun. 2013;4:2250). Because PI3P is a master regulator of endolysosomal function (including retromer function), as well as autophagy, they hypothesized that PI3P deficiency may account for defects in these pathways in AD. To test this and model PI3P deficiency in neurons, they used a combination of pharmacological approaches (i.e., specific Vps34 kinase inhibitors) and mouse genetics (i.e., mice lacking Vps34 selectively in neurons). They observed that disruption of neuronal Vps34 function in vitro and in vivo not only impairs autophagy, lysosomal degradation of APP-CTFs and lipid metabolism, but also causes physical disruption of endolysosomal membranes. The authors also found that PI3P deficiency promotes secretion of unique exosomes enriched for APP-CTFs and the phospholipid BMP, which normally resides in the internal vesicles of endolysosomes. Overall, this study revealed a homeostatic response counteracting lysosomal dysfunction via secretion of atypical exosomes eliminating lysosomal waste and define exosomal APP-CTFs and BMP as candidate biomarkers for endolysosomal dysfunction associated with neurodegenerative disorders. Finally, since exosomes have been found to carry aggregate-prone peptides and/or proteins such as Aβ, tau and α-synuclein, in other instances, the Miranda et al. study raises the possibility that endolysosomal dysfunction may predispose for the exosomal secretion and spreading of pathological proteins within diseased brains.
Gilbert Di Paolo, PhD
Adjunct Associate Professor of Pathology and Cell Biology (in the Taub Institute)
gd2175@cumc.columbia.edu
Denali Therapeutics Inc.
dipaolo@dnli.com
André Miguel Miranda
MD/PhD Student, School of Medicine
School of Medicine, University of Minho
id5609@alunos.uminho.pt
And Graduate School of Arts and Sciences, Columbia University
alm2259@cumc.columbia.edu
 |  | |
| Yian Gu, MD, MS, PhD | Jennifer J. Manly, PhD | |
 |  | |
| Richard Mayeux, MD, MSc | Adam M. Brickman, PhD |
Accumulating evidence from Taub investigators and others suggests that diet may play an important role in the prevention of sporadic, late-onset Alzheimer's disease (AD). Previous work from the longitudinal, population based Washington Heights-Hamilton Heights-Inwood Community Aging Project (WHICAP) cohort, led by Dr. Richard Mayeux, indicated that adherence to a Mediterranean-type diet (MeDi) or other healthy dietary patterns was related with decreased risk for AD. Additional studies of this cohort have found that, among non-demented older adults, such diets were also associated with brain volumes and cognitive performance, two strong predictors for subsequent AD. However, the underlying biological mechanisms for a potentially protective effect of these diets remain unclear.
Among all potential mechanisms, an inflammation pathway might be of particular importance. Studies on the biological effects of individual nutrients or foods have demonstrated that many of them can modulate inflammatory response, and there is strong evidence to suggest the involvement of systemic inflammation in AD or AD-related brain and cognitive decline. To explore mechanisms through which dietary factors may influence cognitive status and dementia risk, Taub investigators Drs. Yian Gu, Jennifer Manly, Richard Mayeux, and Adam Brickman examined data from 330 non-demented elderly participants from WHICAP to see whether an inflammation-related nutrient pattern (INP) was associated with structural MRI findings in the brain and cognitive measures of brain health.
As published in Current Alzheimer Research, the investigators found that an INP, characterized by low intake of calcium, vitamins (D, E, A, B1, B2, B3, B5, B6), folate, and Ω-3 poly-unsaturated fatty acids, and high intake of cholesterol, was positively associated with levels of inflammatory markers (C-reactive protein and interleukin-6). In addition, closer adherence to this INP was associated with smaller total brain volume and worse performance in visuospatial function, independent of age, gender, ethnicity, education, caloric intake, Apolipoprotein E genotype, vascular burden, and head size. Gu et al. further demonstrated that the relationship of this INP and visuospatial cognition could be explained by the differences in brain volume sizes among the population.
These results provide evidence that inflammation may be a potential mechanism through which eating habits might alter brain structure and cognitive function. The authors found evidence to suggest that the magnitude of the effect of consuming a diet that yields a 1-unit higher INP score is comparable to that of 10 years of increasing age. Future studies in larger populations are needed to confirm these findings.
Correspondence to:
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology, and the Taub Institute)
yg2121@cumc.columbia.edu

