Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
August 2018
2: » An Alzheimer's Linked Loss-of-Function CLN5 Variant Impairs Cathepsin D Maturation Consistent with a Retromer Trafficking Defect
3: » Letter and Category Fluency Performance Correlates with Distinct Patterns of Cortical Thickness in Older Adults
A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research

Philip De Jager, MD, PhD, MMSc
The Religious Order Study (ROS) and the Memory and Aging Project (MAP) are two prospective studies of aging that recruit older individuals without known dementia and include (1) detailed cognitive, neuroimaging and other ante-mortem phenotyping and (2) an autopsy at the time of death that includes a structured neuropathologic examination. They were designed to be used in joint analyses to maximize sample size, and for data and sample sharing, and are jointly referred to as "ROSMAP". Cataloguing multi-omic data in all of the ROSMAP subjects regardless of their disease trajectory can provide insight into the molecular events that contribute to aging-related cognitive decline. Taub faculty member Dr. Philip De Jager has been collaborating with the ROSMAP investigators over the past decade, and he has opened a new dimension to these important studies by generating large-scale molecular data from the brains of these subjects. These efforts started with the generation of genetic data and rapidly progressed to yield the most deeply characterized set of aging human brains.
A summary of these efforts was recently published in Scientific Data: Dr. De Jager and colleagues from Taub, along with Dr. David Bennett (Rush Alzheimer Disease Center) and others, report the creation of "A multi-omic atlas of the human frontal cortex for aging and Alzheimer's disease research." This atlas was created from the systematic profiling of the dorsolateral prefrontal cortex obtained from a subset of autopsied individuals enrolled in ROSMAP. They include over 3,322 subjects. The present manuscript outlines the first generation of data, including genome-wide genotypes (n=2,090), whole genome sequencing (n=1,179), DNA methylation (n=740), chromatin immunoprecipitation with sequencing using an anti-Histone 3 Lysine 9 acetylation (H3K9Ac) antibody (n=712), RNA sequencing (n=638), and miRNA profile (n=702).
"The data described in this report represent data that exist today and are available to all investigators on Synapse," explains Dr. De Jager and colleagues. "Numerous additional layers of data, including proteomic and metabolomic data, from tissue samples and purified cell populations are being produced and will become available as the data are finalized. We look forward to this large set of molecular and phenotypic data being repurposed by the neuroscience and other communities of researchers."
Philip De Jager, MD, PhD, MMSc
Weil-Granat Professor of Neurology (in the Taub Institute, the Precision Medicine Initiative, and the Center for Translational and Computational Neuro-immunology)
pld2115@cumc.columbia.edu
 |  |  | ||
| Yasir H. Qureshi, MD | Scott A. Small, MD | Christiane Reitz, MD, PhD |
Lysosomal storage diseases (LSDs) are a group of inherited disorders that typically cause neurodegeneration early in life. Recent observations suggest that genetic variants that cause one type of LSD, Gaucher's disease, can act as a risk factor for developing Parkinson's disease. This observation also suggests that genes that cause other LSDs might act as risk factors for other late-onset neurodegenerative disorders, notably Alzheimer's disease (AD).
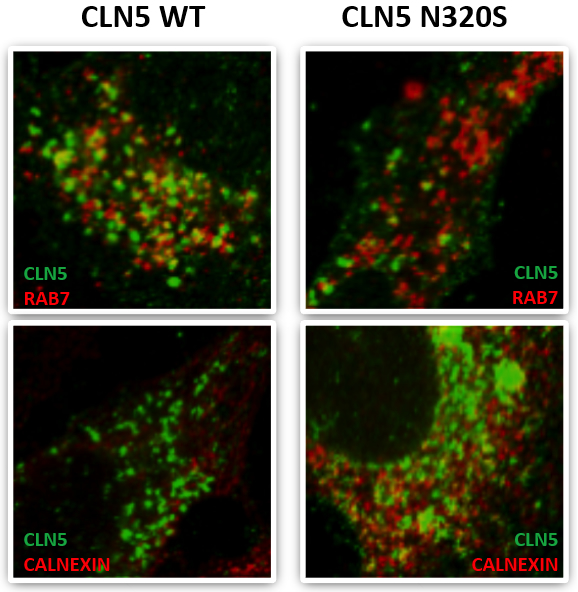
Figure: CLN5 colocalization with the late endosomal marker RAB7 and the ER maker Calnexin.
Published recently in Molecular and Cellular Biology, a new study by Drs. Scott Small and Christiane Reitz, with first author Dr. Yasir Qureshi and colleagues, focused on a select group of genes that cause neuronal ceroid lipofuscinosis (CLN3, CLN5, Cathepsin D) and have been directly or indirectly associated with retromer trafficking, which is also involved in the pathogenesis of late-onset AD. Capitalizing on whole exome sequencing data from multiplex AD families from the EFIGA Study, they identified a missense variant in CLN5 c.A959G (p.Asn320Ser) that segregated with AD and caused glycosylation defects in the expressed protein, causing the protein to be retained in the endoplasmic reticulum with reduced delivery to the endolysosomal compartment, CLN5’s normal cellular location. They further showed that the AD-associated CLN5 variant reduced the normal processing of Cathepsin D and was associated with decreased levels of full-length APP, consistent with a defect in retromer-dependent trafficking.
Yasir H. Qureshi, MD
yhq2001@cumc.columbia.edu
Postdoctoral Research Scientist (in the Taub Institute)
Scott A. Small, MD
Boris and Rose Katz Professor of Neurology (in the Taub Institute, the Sergievsky Center, Radiology, and Psychiatry)
sas68@cumc.columbia.edu
Christiane Reitz, MD, PhD
Assistant Professor of Neurology and Epidemiology (in the Gertrude H. Sergievsky Center and the Taub Institute)
cr2101@cumc.columbia.edu
 |  | |
| Jet M. J. Vonk, PhD | Adam M. Brickman, PhD |
Verbal fluency tasks—naming as many items of a given criterion, typically letters or categories, under time constraints—are one of the cornerstones of the prevailing neuropsychological battery and, as such, are widely administered in both research and clinical settings. In general, letter fluency is more strongly linked to executive function abilities mediated by frontal brain regions, while category fluency relies more strongly on verbal abilities mediated by temporal regions. This idea, however, is primarily based on lesion studies and adapted versions of the fluency tasks in functional neuroimaging, without fundamental evidence from structural neuroimaging in healthy individuals.
A new study by Dr. Adam Brickman, postdoctoral fellow and first author Dr. Jet Vonk from the laboratory of Dr. Jennifer Manly, and colleagues from Taub investigated the cortical structural correlates of letter and category fluency, including overlapping and different regions, in 505 individuals from the Washington Heights-Inwood Columbia Aging Project (WHICAP). As published recently in Cerebral Cortex, they found that the correlation between cortical thickness and verbal fluency in whole-brain analyses revealed distinct cortical signatures for letter fluency, primarily in frontal regions, and category fluency, in frontal and temporal-parietal regions. "By elucidating cortical signature of each fluency task," Vonk et al. conclude, "we can further build our understanding of the meaning of verbal fluency scores in various clinical populations and the theoretical mechanisms of executive functions and lexical processing."
Jet M. J. Vonk, PhD
Postdoctoral Research Scientist (in the Gertrude H. Sergievsky Center)
jv2528@cumc.columbia.edu
Adam M. Brickman, PhD
Associate Professor of Neuropsychology (in Neurology, the Taub Institute and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu