Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2017
#2 Association of Obstructive Sleep Apnea with Episodic Memory and Cerebral Microvascular Pathology: A Preliminary Study
Local Synthesis of Dynein Cofactors Matches Retrograde Transport to Acutely Changing Demands
 |  | |
| Ulrich Hengst, PhD | Joseph M. Villarin, PhD |
Retrograde transport from axons to neuronal cell bodies is essential for neuronal development and function, and disruption of this process is associated with neurodevelopmental disorders and neurodegeneration. All microtubule-based retrograde transport in axons is mediated by a single motor, cytoplasmic dynein. An array of cofactors, regulators or cargo adaptors allows dynein to fulfill a multitude of functions and to transport many different cargos. How dynein-dependent transport is regulated by these proteins and how this process is controlled by the cell continues to be a very active field of research.
The unidirectional orientation of microtubules in axons necessitates that dynein is transported as an inactive cargo into the periphery, leading to the deceptively simple question that provoked the present study: how is the dynein motor activated and tuned for specific transport needs in distal axons? Published in Nature Communications, Taub faculty member Dr. Ulrich Hengst and members of his laboratory, along with first author and former graduate student Dr. Joseph Villarin, report that extracellular stimuli can activate the transport of specific cargos by triggering the local synthesis of those dynein regulators that are required for these specific cargos. NGF-induced transport of large cargos required local synthesis of Lis1 while smaller NGF-signaling endosomes required both Lis1 and the dynactin subunit p150Glued. Surprisingly, the removal of NGF also triggered local synthesis of Lis1, but in this context, it was required for the retrograde transport of a pro-apoptotic death signal. Villarin et al. further discovered that while NGF stimulation and deprivation both triggered Lis1 synthesis, they actually act on functionally distinct pools of axonally localized Lis1 transcripts that differ in their association with the RNA-binding protein APC.
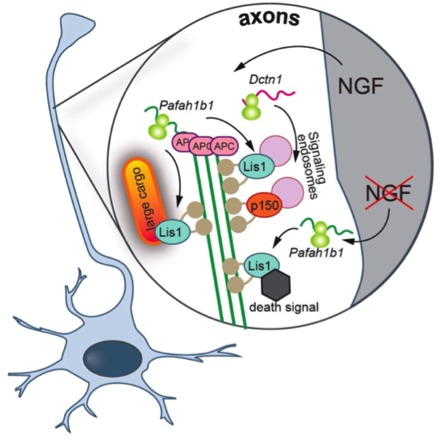
Proposed model: NGF stimulation leads to the axonal translation of Dctn1 and APC-bound Pafah1b1. Locally synthesized Lis1 required for the retrograde transport of vesicular cargoes greater than 1 ╬╝m in diameter, and both axonally-derived Lis1 and p150Glued are necessary for the retrograde transport of NGF-signaling endosomes. NGF withdrawal has no effect on p150Glued synthesis but causes the production of Lis1 off not APC-bound Pafah1b1 transcripts. Lis1 produced in response to NGF withdrawal is required for the retrograde transport of a death signal (from: Villarin, J.M., McCurdy, E.P., Mart├şnez, J.C., and Hengst, U. (2016). Local synthesis of dynein cofactors matches retrograde transport to acutely changing demands. Nat Commun 7, 13865)
The present study includes several conceptual advances for the understanding of intra-axonal transport. The tuning of the retrograde transport complex through the on-demand production of Lis1 or p150Glued provides a mechanistic explanation for how extracellular signals can acutely activate dynein and trigger the transport of different cargos in axons. They found that NGF deprivation triggered local Lis1 production that is required for the retrograde transport of a death signal. Dr. Hengst and colleagues described this finding as "exciting because it lends strong support for the dependence receptor hypothesis of TrkA receptor function. Rather than solely providing trophic support through the transport of NGF-signal endosomes from axons to cell bodies, our data suggest that NGF-binding to TrkA represses a cell intrinsic death signaling pathway downstream of TrkA, which we propose to be active GSK3╬▓." Currently, it remains unknown how specificity in local translation is achieved. Only a certain set of localized mRNAs is translated in response to a specific stimulus, and many mRNAs react to more than one stimulus. Villarin et al. report that some Lis1 transcripts are axonally localized in an APC-dependent mode, making them responsive to NGF stimulation, while a second APC-independent pool of Lis1 is recruited into axons and locally translated only in NGF-starved axons. Their findings establish that the association with structural RNA-binding proteins such as can be a mechanism to establish functionally specialized pools of the same transcript species within axons.
In ongoing research, Dr. Hengst and colleagues are investigating whether similar mechanisms affect retrograde transport under neurodegenerative conditions, as part of a larger project to characterize the molecular mechanisms underlying the long-range spread of pathology in AD brain.
Ulrich Hengst, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute)
uh2112@columbia.edu
Joseph M. Villarin, PhD
Student, Medical Scientist Training Program
jmv2127@columbia.edu
 |  | |
| Davangere P. Devanand, MBBS, MD | Nancy Kerner, MD |
Obstructive sleep apnea (OSA) is a primary sleep disorder caused by repeated partial or complete upper airway collapse, despite an ongoing effort to breathe during sleep. Epidemiological studies report that individuals with OSA have a high prevalence of depression and cognitive impairment as compared to the general population. Current structural imaging data suggest that hippocampal atrophy and microvascular damage, including white matter hyperintensities, white matter integrity abnormalities, and gray matter loss, are accompanied by OSA. Although OSA is known for its destructive nature in the cerebral vascular and central neural systems, whether OSA plays an important role in the cognitive deteriorating process in older adults with amnestic mild cognitive impairment (aMCI) and depression is not clear. No prior study has evaluated the impact of OSA in this population.
In a sub-study of the Donepezil Treatment of Older Adults with Cognitive Impairment and Depression (DOTCODE) trial, led by Taub faculty members Drs. Davangere P. Devanand and Gregory Pelton, Dr. Nancy Kerner (an NIMH-funded T32 research fellow) and colleagues from the Division of Geriatric Psychiatry examined the association between OSA, cognition, and brain structural abnormalities in older adults with aMCI and depression. The investigators used the widely validated STOP-Bang questionnaire as an add-on instrument to assess the probability of OSA. Using standard methods, a score of 5 or greater was defined as a high probability of OSA (h-OSA), and a score of less than 5 was defined as a low probability of OSA (l-OSA). Baseline magnetic resonance imaging (MRI) was used to evaluate brain morphology. The initial 16 weeks of antidepressant treatment were part of the DOTCODE trial.
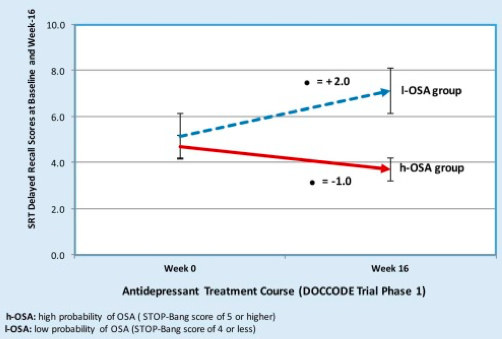
Figure 1. Comparison performance on delayed recall tasks following antidepressant treatment between high and low probability of obstructive sleep apnea. h-OSA: high probability of OSA (STOP-Bang score of 5 or higher); l-OSA: low probability of OSA (STOP-Bang score of 4 or less).
Kerner et al. reported their preliminary findings in the American Journal of Geriatric Psychiatry this month, which included 1) a significant association between OSA and severity of microvascular damage as identified on MRI, 2) no significant association between OSA and hippocampal or entorhinal cortex volume, even after controlling for intracranial volume, and 3) following 16 weeks of antidepressant treatment, no statistically significant changes in cognitive measures across multiple domains between the groups, with the exception of verbal episodic memory. One possible explanation for these findings is that OSA may facilitate additional neural injury (i.e., beyond that which is seen in AD pathology alone) and accelerate cognitive decline. Consequently, cognitive deficits accompanying OSA may persist or progress if OSA is left untreated.
Davangere P. Devanand, MD
Professor of Psychiatry (in Neurology and the Gertrude H. Sergievsky Center)
dpd3@cumc.columbia.edu
Nancy Kerner, MD
Instructor in Clinical Psychiatry
nak2120@cumc.columbia.edu

