Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Ulrich Hengst, PhD

Ulrich Hengst, PhD
In our research group, we are interested in compartmentalized signaling events in neurons. Specifically, we are investigating how intra-axonal signaling and the localized proteome are changed in response to extracellular signals or neurodegenerative insults and how these localized signaling events retrogradely affect the entire neuron. Axonal mRNA translation is crucial for axonal pathfinding and maintenance during the development of the nervous system, but so far very little is known about its role in the mature brain or in the context of age-related neurodegenerative disorders. We study neurodevelopment and neurodegeneration in parallel to gain insight into commonalities and differences between both processes. A core technique in our group is the use of primary neurons cultured in microfluidic chambers that allow us to specifically study and interfere with intra-axonal signaling events. Overall, our aim is to understand the unique properties of axonal protein synthesis and to investigate whether targeting the axonal translatome might offer a new way to slow the propagation of disease across the brain.
Currently, our research efforts are focused on two main projects: (1) the role of intra-axonal protein synthesis in developing and regenerating axons and (2) the reactivation of local translation in AD.
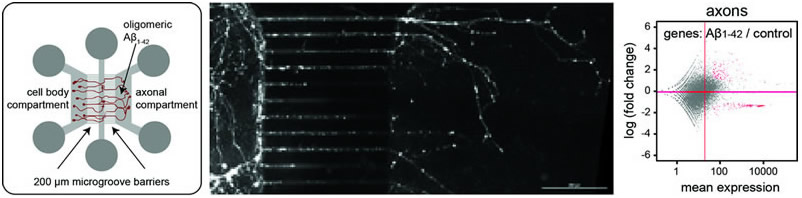 (left) Schematic drawing of microfluidic chamber used to treat axons selectively with oligomeric ╬▓-amyloid. (middle) Neurons cultured in compartmentalized chambers with isolated axons on the right side of the microgroove barrier. (right) Changes of the axonal transcriptome in response to A╬▓. |
Jimena Baleriola is leading a project addressing the emerging role of intra-axonal protein synthesis in the context of neurodegenerative disorders. She is using a wide range of techniques from compartmentalized neuronal primary cultures, RNA sequencing (in collaboration with Peter Nagy), mouse injection models (with Carol Troy's group), and investigation of human post-mortem tissue (with John Crary) to determine how changes in the axonal translatome, in response to axonal exposure to ╬▓-amyloid, contribute to neurodegeneration. Currently, she aims to characterize intra-axonal stress response signaling as an important driver of Alzheimer's disease pathology.
Chandler Walker is interested in the earliest signaling events triggered within axons in response to oligomeric ╬▓-amyloid. She is especially focused on the question of how the neurodegenerative stimulus is communicated to the neuronal soma and whether interference with this communication might slow down the spread of pathology in Alzheimer's disease brain.

Members of the Hengst laboratory, from left to right, Chandler Walker; José Mart├şnez; Jimena Baleriola, PhD; Neilia Gracias, PhD; Joseph Villarin.
Neilia Gracias is leading our research efforts at the developmental side. Recently, she established that local protein synthesis is a molecular mechanism to ensure synchronistic and spatial coherence between cytoskeletal elongation and membrane expansion in rapidly growing axons. Currently, she is investigating the crosstalk between two multi-protein complexes, the PAR complex and the exocyst, that regulate cytoskeleton and membrane dynamics, respectively.
José Martínez combines bioinformatics and molecular biology approaches to investigate a molecular mechanism that controls the axonal localization of a specific class of mRNAs. He has identified a cis-acting RNA regulatory element and investigates how binding of this element to RNA-binding proteins determines the fate of mRNAs within axons and cell bodies.
Joseph Villarin is investigating how the transport characteristics and cargo preferences of the unidirectional motor dynein is acutely adjusted to changing transport needs in developing axons.