Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2021
2: Pathogenic Role of Delta 2 Tubulin in Bortezomib-Induced Peripheral Neuropathy
 |  |  | ||
| Adam M. Brickman, PhD | Jennifer J. Manly, PhD | Richard Mayeux, MD, MSc |
Blood-based biomarkers can be easily and routinely obtained, even among elderly or frail populations, and can be analyzed quickly and cost-effectively with high-throughput assays that rely on rapidly developing technologies. As such, blood-based biomarkers for AlzheimerÔÇÖs disease (AD) provide unique opportunities for community studies that examine participants from diverse race and ethnicity backgrounds, where they have the potential to identify AD at an early stage, and to extend knowledge of the underlying disease pathogenesis.
A new study by Drs. Richard Mayeux, Adam Brickman, Jennifer Manly, and colleagues from Taub used stored plasma to measure blood-based biomarkers from the Washington Heights-Inwood Columbia Aging Project (WHICAP), a multiethnic community study of aging and dementia. In collaboration with colleagues from Eli Lilly, their focus was on current, stateÔÇÉofÔÇÉtheÔÇÉart ADÔÇÉrelated plasma biomarkers, including amyloid beta (A╬▓)40 and A╬▓42 as markers of amyloid pathology, total tau (tÔÇÉtau) and neurofilament light (NfL) chain as markers of neurodegeneration, and phosphorylated tau (pÔÇÉtau) 181 and 217 as markers of tau pathology. Brickman et al. examined how well plasma biomarker concentrations discriminated between clinically and pathologically defined diagnostic groups. A subset had undergone florbetaben PET to assess cortical A╬▓ plaque burden.
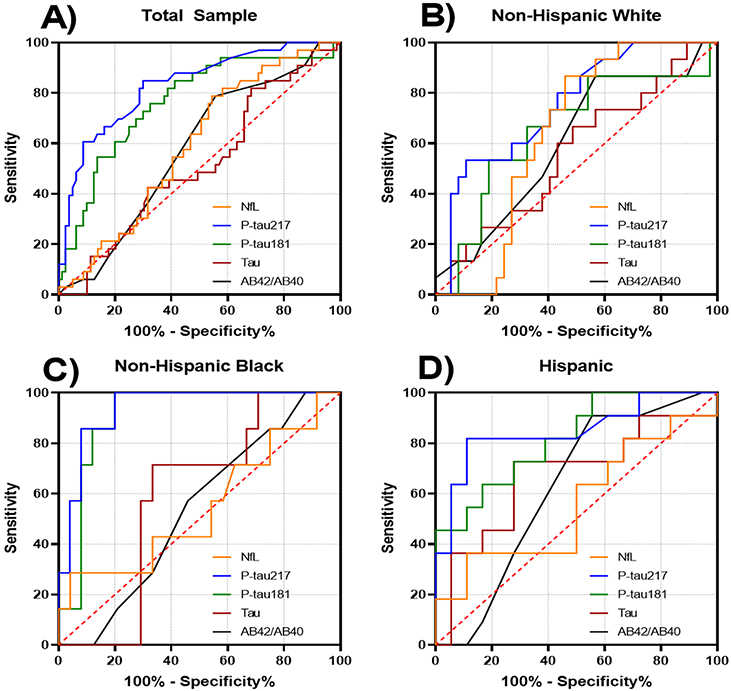 Figure 1: Receiver operating curves for classification of post mortem diagnosis of Alzheimer's disease. A, Total sample. B, NonÔÇÉHispanic Whites. C, NonÔÇÉHispanic Blacks. D, Hispanics
|
As recently reported in AlzheimerÔÇÖs & Dementia, they found that the plasma biomarker concentrations of phosphorylated tau, particularly pÔÇÉtau217, were strongly associated with autopsyÔÇÉconfirmed AD. PÔÇÉtau biomarkers were also associated with amyloid pathology on PET, more so than other plasma biomarkers, including A╬▓42/A╬▓40. Among individuals classified as controls at time of blood draw, lower A╬▓42/A╬▓40 ratio, and higher pÔÇÉtau217 or pÔÇÉtau181 concentrations, were associated with increased risk of subsequent AD diagnosis.
According to Brickman et al., plasma levels of pÔÇÉtau217 outperformed all other biomarkers across analyses, achieving AUCs of 0.84 overall, and 0.85 and 0.96 in Hispanic and Black participants with pathological diagnosis. In the analysis of those who developed subsequent AD dementia, both pÔÇÉtau181 and pÔÇÉtau217 as well as the A╬▓42/A╬▓40 ratio were reliable predictors.
Taken together, their results provide encouraging data for the use of bloodÔÇÉbased biomarkers in diverse cohorts across clinical settings and in observational, epidemiological studies, but also highlight the need to understand the moderators and mediators of the relationship between biomarkers concentrations and diagnosis in community-dwelling older adults.
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Taub Institute and the Gertrude H. Sergievsky Center)
rpm2@cumc.columbia.edu
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
Jennifer J. Manly, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute and the Gertrude H. Sergievsky Center)
jjm71@cumc.columbia.edu
Pathogenic Role of Delta 2 Tubulin in Bortezomib-Induced Peripheral Neuropathy
 |  | |
| Maria Elena Pero, DVM, PhD | Francesca Bartolini, PhD |
Several classes of anticancer drugs with different antineoplastic mechanisms have been shown to induce chemotherapy-induced peripheral neuropathy (CIPN), a debilitating distal-to-proximal degenerative nerve disorder, the pathogenic mechanisms of which remain largely unknown. Tubulin and microtubules are well-established targets for multiple anticancer drugs that induce CIPN. The contribution of the microtubule changes to the onset of CIPN is not well understood but is strongly implicated as the determining factor. With support from the Thompson Family Foundation InitiativeÔÇöa unique, multidisciplinary research initiative focused on CIPN and sensory neuroscienceÔÇöa new study from the laboratory of Taub faculty affiliate Dr. Francesca Bartolini sought to test the hypothesis that seemingly unrelated CIPN-causing drugs share a pathogenic mechanism by converging on alterations of the tubulin cytoskeleton.
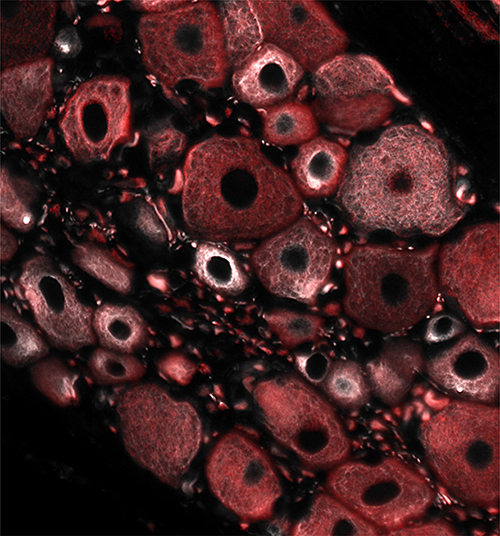
Figure 1: Immunofluorescence image of dorsal root ganglion (DRG) neurons dissected from a rat treated with an acute dose of the proteasome inhibitor bortezomib and stained for D2-tubulin (white) and bIII-tubulin (red)
Image credit: Maria Elena Pero and Francesca Bartolini (Columbia University in the City of New York, USA)
Now published in PNAS and featured on the CUIMC Newsroom, Dr. Bartolini and colleagues, including first author Dr. Maria Elena Pero, report that bortezomib, a CIPN-causing drug used to treat multiple myeloma, promotes delta 2 tubulin (D2) accumulation while affecting microtubule stability and dynamics in sensory neurons in vitro and in vivo. Importantly, the authors find that accumulation of D2 is predominant in unmyelinated fibers and a hallmark of bortezomib-induced peripheral neuropathy (BIPN) in humans. In addition, while D2 overexpression is sufficient to cause axonopathy and inhibit mitochondria motility, reduction of D2 levels alleviates both axonal degeneration and the loss of mitochondria motility induced by bortezomib.
Together, these findings from Pero et al. demonstrate that bortezomib, a compound structurally unrelated to tubulin poisons, affects the tubulin cytoskeleton in sensory neurons in vitro, in vivo, and in human tissue, indicating that the pathogenic mechanisms of seemingly unrelated CIPN drugs may converge on tubulin damage.
Altogether, these results reveal a previously unrecognized pathogenic role for D2 in BIPN that may occur through altered regulation of mitochondria motility. Further, their data provide a strong rationale for pursuing the potential of targeting tubulin modifying enzymes of the tyrosination/de-tyrosination cycle in drug therapies aimed at preventing and/or rescuing axonal injury observed in BIPN and perhaps related neuropathies.
Francesca Bartolini, PhD
Associate Professor of Pathology and Cell Biology at CUIMC
fb2131@cumc.columbia.edu
Maria Elena Pero, DVM, PhD
Associate Research Scientist (in Bartolini Lab)
mp3054@cumc.columbia.edu

