Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2019
2: »Brain Arterial Dilatation and the Risk of Alzheimer's Disease
 |  | |
| Andrew F. Teich, MD, PhD | Lawrence S. Honig, MD, PhD |
Normal pressure hydrocephalus (NPH) is a condition usually found in the elderly and is characterized by gait dysfunction, cognitive decline, and urinary incontinence. NPH patients typically undergo a trial of cerebrospinal fluid (CSF) drainage over several days to assess improvement in each of the three major symptom groups. Gait and balance are the most common symptoms to improve, whereas cognition is generally recognized as the least likely symptom to improve in NPH. While it is well known that NPH patients can cognitively improve after permanent ventriculoperitoneal shunting (VPS), one of the major dilemmas in NPH is the ability to predict which patients will improve. Currently, there are no guidelines regarding the cognitive evaluation of these patients.
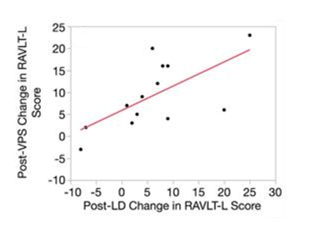
Figure 2B: Rey Auditory Verbal Learning Test-Learning (RAVLT-L) scores for NPH patients. B, Post-VPS RAVLT-L improvement is correlated with post-LD RAVLT-L improvement in a linear fashion (R2 = 0.43, P = .015).
Thus, in collaboration with Dr. Guy McKhann and colleagues from the Department of Neurosurgery, Taub faculty members Drs. Andrew Teich and Lawrence Honig sought to determine if improvement on neuropsychological testing after CSF lumbar drainage could predict post-VPS cognitive improvement. To do this, they used a short battery of neuropsychological tests, which included the Rey Auditory Verbal Learning Test (RAVLT). A recent meta-analysis has demonstrated that NPH patients consistently demonstrate improvement on the RAVLT-L (short/immediate term recall) and RAVLT-D (delayed recall) post-VPS. Although this was the primary question asked in this study, the authors also examined if the absence of neurodegenerative pathology on cortical biopsies taken from NPH patients could predict post-VPS cognitive improvement. Finally, they investigated whether they could predict a patient's cortical biopsy result based solely on preoperative CSF biomarkers.
As published recently in Neurosurgery, their prospective observational study demonstrates that the RAVLT can be a useful preoperative predictor of postoperative cognitive improvement. NPH patients performed significantly better on the RAVLT-L after lumbar CSF drainage (LD). An individual patient's change in RAVLT-L score post-LD correlated with his/her change in RAVLT-L score post-VPS. In addition, unlike some prior studies that have shown that post-VPS improvement relies mainly on baseline cognitive function, they did not see any differences in baseline RAVLT-L scores between NPH responders and non-responders. This indicates that even patients with relatively poor baseline cognition have the ability to improve after VPS as long as they demonstrate improvement post-LD. The study with biopsy and CSF was less definitive. There was a relationship between CSF and biopsy results; patients with biopsies demonstrating Aβ+ Tau+ had lower ventricular CSF Aβ42 and higher lumbar CSF pTau compared to Aβ– Tau– patients. In addition, a receiver operating curve analysis using lumbar pTau predicted Aβ+ Tau+ biopsy status. However, neither biopsy status nor CSF Aβ42, Tau, or pTau status related to neuropsychological test outcome. Of note, the sample size of this study may have been too limited to find a relationship between biopsy/CSF status and neuropsychological test outcome, and future studies may be necessary to fully determine this relationship.
In summary, the authors propose that the first priority in evaluating an NPH patient should be RAVLT testing. If the patient improves by approximately 5 to 10 words on the RAVLT-L portion after lumbar CSF drainage, one can likely expect post-VPS cognitive improvement regardless of other factors.
Andrew F. Teich, MD, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
aft25@cumc.columbia.edu
Lawrence S. Honig, MD, PhD
Professor of Neurology (in the Gertrude H. Sergievsky Center and the Taub Institute) at CUIMC
lh456@cumc.columbia.edu
Brain Arterial Dilatation and the Risk of Alzheimer's Disease
 |  | |
| Jose Gutierrez-Contreras, MD, MPH | Adam M. Brickman, PhD |
Brain arterial diameters are imaging biomarkers of vascular health. In his ongoing research on brain arterial remodeling in the context of atherosclerosis and aging, Columbia Neurologist Dr. Jose Gutierrez has shown that very small or very large brain arterial diameters are pathological, and predict higher vascular risks. Dr. Gutierrez has also found that larger brain arterial diameters (measured by MRI) relate to poorer cognitive performance and greater cognitive decline over time. Further, in previous research co-authored by Taub faculty member Dr. Lawrence Honig, Dr. Gutierrez also demonstrated evidence of greater brain arterial aging in Alzheimer’s disease (AD) patients, independent of chronological age, brain infarction, and large artery atherosclerosis, compared with non-AD cases.
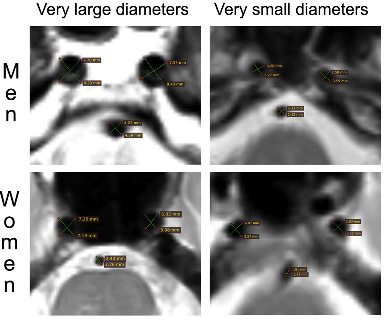
Figure 1: Example of MRI measurements of brain arterial diameters. Gutierrez et al. measured the cross-sectional axial voids in brain MRI T2 sequences. Each artery was measured twice as demonstrated by the green lines intersection each round void. The two measurements in each artery where averaged to account for naturally occurring curvature and circular deformation of brain arteries as they course through the subarachnoid space intracranially.
Understanding the role that brain arterial dilatation plays in cognitive decline and/or Alzheimer’s disease (AD) may uncover new pathways to reduce the risk of dementia. In his latest research, Dr. Gutierrez collaborated with Taub faculty member Dr. Adam Brickman to investigate brain arterial dilatation as a potential MRI risk factor for AD. Now published online in Alzheimer’s & Dementia, Drs. Gutierrez, Brickman, and co-investigators from Neurology and Taub examined dementia-free participants from the multiethnic Washington Heights-Inwood Community Aging Project cohort and found that those with larger intracranial carotid arteries were at a higher risk of AD independent of vascular risk, silent brain infarcts, and white matter disease. There were no differences in risk by APOE status.
Gutierrez et al. conclude that the association between dilated intracranial carotid arteries and AD may imply a role in the pathogenesis, though further work is needed to establish such a causal inference. With his recent receipt of a NIH R01 award titled, "Genetic Contribution to Brain Arterial Dilatation and its Role in Cognition and Dementia," Dr. Gutierrez plans to extrapolate this research to >6,000 participants worldwide.
Jose Gutierrez-Contreras, MD, MPH
Assistant Professor of Neurology
jg3233@cumc.columbia.edu
Adam M. Brickman, PhD
Associate Professor of Neuropsychology
amb2139@cumc.columbia.edu

