Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
July 2020
2: » Proteomic Profiles for Alzheimer's Disease and Mild Cognitive Impairment Among Adults with Down Syndrome Spanning Serum and Plasma: An Alzheimer's Biomarker Consortium-Down Syndrome (ABC-DS) Study
3: » Cognitive Tests aid in Clinical Differentiation of Alzheimer's Disease Versus Alzheimer's Disease with Lewy Body Disease: Evidence from a Pathological Study
Endothelial Activation of Caspase-9 Promotes Neurovascular Injury in Retinal Vein Occlusion
Members of the Troy Laboratory (from left to right), 1st row: Maria Avrutsky, Carol Troy (PI), Crystal Colon Ortiz; 2nd row: Kendra Johnson, Anna Potenski, Jackie Lawson; 3rd row: Monica Choi, Crystal Collier, James Belarde; 4th row: Jade Smart, Claire Chen
Regulation of the neurovascular unit is an active area of research interest in multiple disciplines because many neurologic disorders exhibit both neuronal dysfunction and loss of vascular integrity. However, the mechanistic relation of these phenomena has not been explicated. Studies have been hampered by the lack of available animal models that enable real-time live imaging of the neurovascular unit. Utilizing in vivo imaging of the mouse retina, Dr. Carol Troy and colleagues, including first author Dr. Maria Avrutsky, have investigated the mechanistic relation of vascular and neuronal injury, employing a mouse retinal vein occlusion (RVO) model of hypoxia-ischemia.
Dr. Troy has an enduring interest in understanding the role of the caspase family of proteases in the mature nervous system. Her group’s recent publication in Nature Communications establishes caspase-9 mediated endothelial cell signaling as the pivotal event in the subsequent loss of vascular integrity and neuronal dysfunction. Caspase-9 activity has been tightly associated with the initiation of the intrinsic apoptosis pathway, and far less is known about caspase biology in nonapoptotic processes. This work shows that endothelial cells play an active role in maintaining vascular and neuronal health, and that nonapoptotic activation of caspase-9 in endothelial cells promotes neurodegeneration.
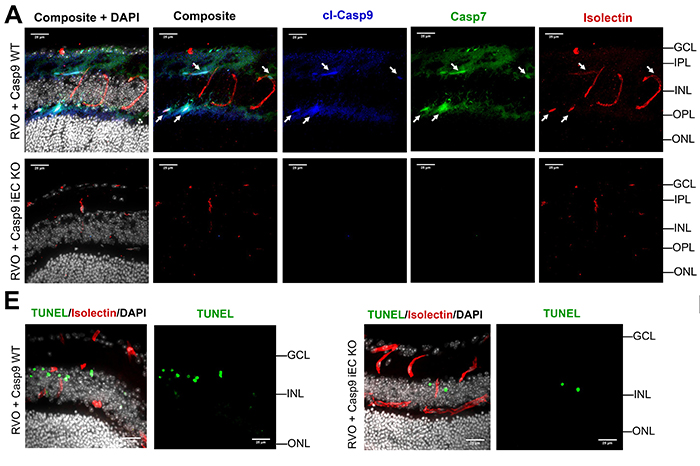 Figure 7: Endothelial caspase-9 mediates neuronal injury following RVO
A. Retinal cross-sections 24hr post-RVO from Casp9 WT and Casp9 iEC KO mice immunostained for cl-caspase-9 (blue), caspase-7 (green), isolectin (red) and DAPI (white). White arrows = vessels expressing cl-caspase-9 and caspase-7. Scale bar=25µm. E. Retinal cross-sections of Casp9 WT (n=11) and Casp9 iEC KO (n=6) eyes 24hr post-RVO, stained for TUNEL (green), isolectin (red) and DAPI. cl-casp9, cl-caspase-9; casp7, caspase-7. Source data are provided as a Source Data file. |
As recently featured on the CUIMC Newsroom, this work also provides a translational approach to developing interventions for neurovascular dysfunction: the authors identify a tractable target for treatment of hypoxic and ischemic injury, use clinically relevant ophthalmic imaging to track changes in retinal pathology, and demonstrate topical drug delivery to the retina via eye drops. Topical drug delivery presents a potential paradigm shift for treatments of retinal disease, since current therapies require clinician-administered intravitreal injections.
By correlating clinically-relevant measures of retinal injury and immunohistochemical analysis, the authors demonstrate how the retina is a powerful in vivo model to study regulation of neuronal health and barrier integrity. This model allows examination of the neurovascular unit in a physiologic setting, providing ease of use and translational relevance. They also demonstrate a simple-to-use and noninvasive strategy to deliver pharmacologic interventions into retinal tissues. This approach can be applied to a wide variety of proteins and peptides, and may be of interest to basic scientists as a research tool to interrogate the neurovascular unit and to clinicians as a potential noninvasive therapeutic.
Carol M. Troy, MD, PhD
Professor of Pathology and Cell Biology and Neurology (in the Taub Institute)
cmt2@cumc.columbia.edu
 |  | |
| Nicole Schupf, PhD | Joseph H. Lee, DrPH |
The use of blood-based biomarkers as a screening tool for the presence of neurodegenerative disease have gained considerable support for application in adults with Down syndrome (DS) for being less invasive and burdensome in clinical practice and clinical trials. Unlike in the general AD population where a serum proteomic profile has been successfully validated, only one study has examined the utility of blood biomarkers in adults with DS.
A new study co-authored by Taub faculty members Drs. Nicole Schupf and Joseph Lee, led by Dr. Sid O’Bryant of the University of North Texas Health Sciences Center, is the first to apply a panel of inflammatory proteomic markers to adults with DS. As recently published by Petersen et al. in Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, their results reveal that the proteomic profiles derived from both serum and plasma produced excellent detection accuracy to distinguish prevalent MCI and AD from those without any signs of dementia. Detection accuracy reached an area under the curve (AUC) ranging from 95% to 98% for serum and plasma proteomic profiles for distinguishing those with MCI and AD from those without. The rates of accuracy were comparable to those observed in the general population.
This study identified sets of inflammatory proteomic markers (e.g., B2M, TNF-𝛼, IL-5, IL-10, CRP, IL-18, and IL-6) from serum and plasma that distinguished those with MCI-DS and DS-AD from those who were cognitively stable. Current work is focused on developing preclinical proteomic profiles to predict incident MC-DS and DS-AD to allow for future therapeutic interventions before irreversible cognitive deterioration has occurred.
Nicole Schupf, PhD
Professor of Epidemiology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
ns24@cumc.columbia.edu
Joseph H. Lee, DrPH
Professor of Epidemiology (in the Gerturde H. Sergievsky Center and Taub Institute)
jhl2@cumc.columbia.edu
 |  | |
| Stephanie Cosentino, PhD | Yaakov Stern, PhD |
Although Alzheimer’s disease (AD) and Lewy body disease (LBD) have unique pathological profiles, patients commonly present with pathologies characteristic of both diseases. Clinical differentiation between AD versus AD plus LBD remains relatively imprecise. Drs. Stephanie Cosentino, Yaakov Stern, Yian Gu, Sylvia Chapman, and colleagues sought to further the understanding of neuropsychological differences across AD versus AD plus LBD by examining group differences in performance across a range of neuropsychological tests. These included measures of visuoconstruction, processing speed, memory, language, attention, and executive functioning in 51 participants with postmortem diagnoses of AD (n=34) versus AD plus LBD (n=17). They further assessed the ability of tests that differed at the group level to classify individuals into pathologic group, while accounting for noncognitive symptoms (i.e., hallucinations and extrapyramidal signs) that are often present in individuals with AD who have comorbid LBD.
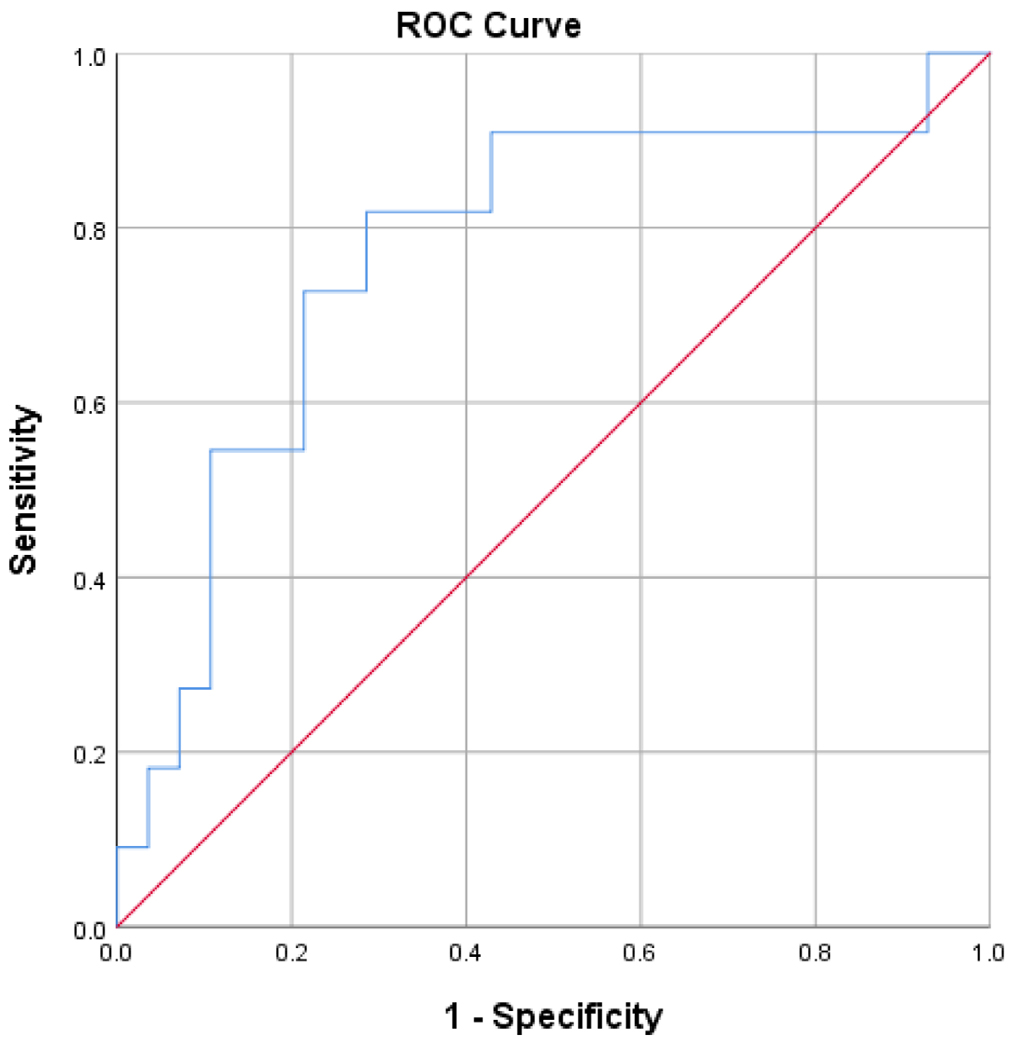
Figure 4: ROC curve of processing speed and visuoconstruction measures
As recently reported in Alzheimer’s & Dementia, they found that AD plus LBD was characterized by relatively greater impairments in executive functioning, processing speed, and visuoconstructional abilities. Groups did not differ in memory or semantic processing. Their results offer guidance in the selection and interpretation of neuropsychological tests to be used in the differential diagnosis of early dementia.
Stephanie Cosentino, PhD
Associate Professor of Neuropsychology (in Neurology, the Gertrude H. Sergievsky Center and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
sc2460@cumc.columbia.edu
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center, and in the Taub Institute)
ys11@cumc.columbia.edu

