Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
December 2019
2: » Role of Tau Protein in Remodeling of Circadian Neuronal Circuits and Sleep
3: » Sleep Fragmentation, Microglial Aging, and Cognitive Impairment in Adults with and Without Alzheimer's Dementia
 |
 |
| Xiaoyi (Lily) Qu, PhD | Francesca Bartolini, PhD |
Dynamic microtubules (MTs) play a crucial role for rapid forms of axonal and dendritic transport and synaptic transmission. At postsynaptic sites, MT invasion into spines regulates spine morphology, motor and cargo pair transport into spines, and synaptic activity. MT dynamics and organization at presynaptic boutons remains largely unexplored, however, due to the limited size of most mammalian boutons and the poor resistance of dynamic microtubules to standard fixation procedures. Therefore, it remains unknown whether axonal de novo MT nucleation occurs post-development, is required for neurotransmission, and is a feature of neurons residing in an intact circuit.
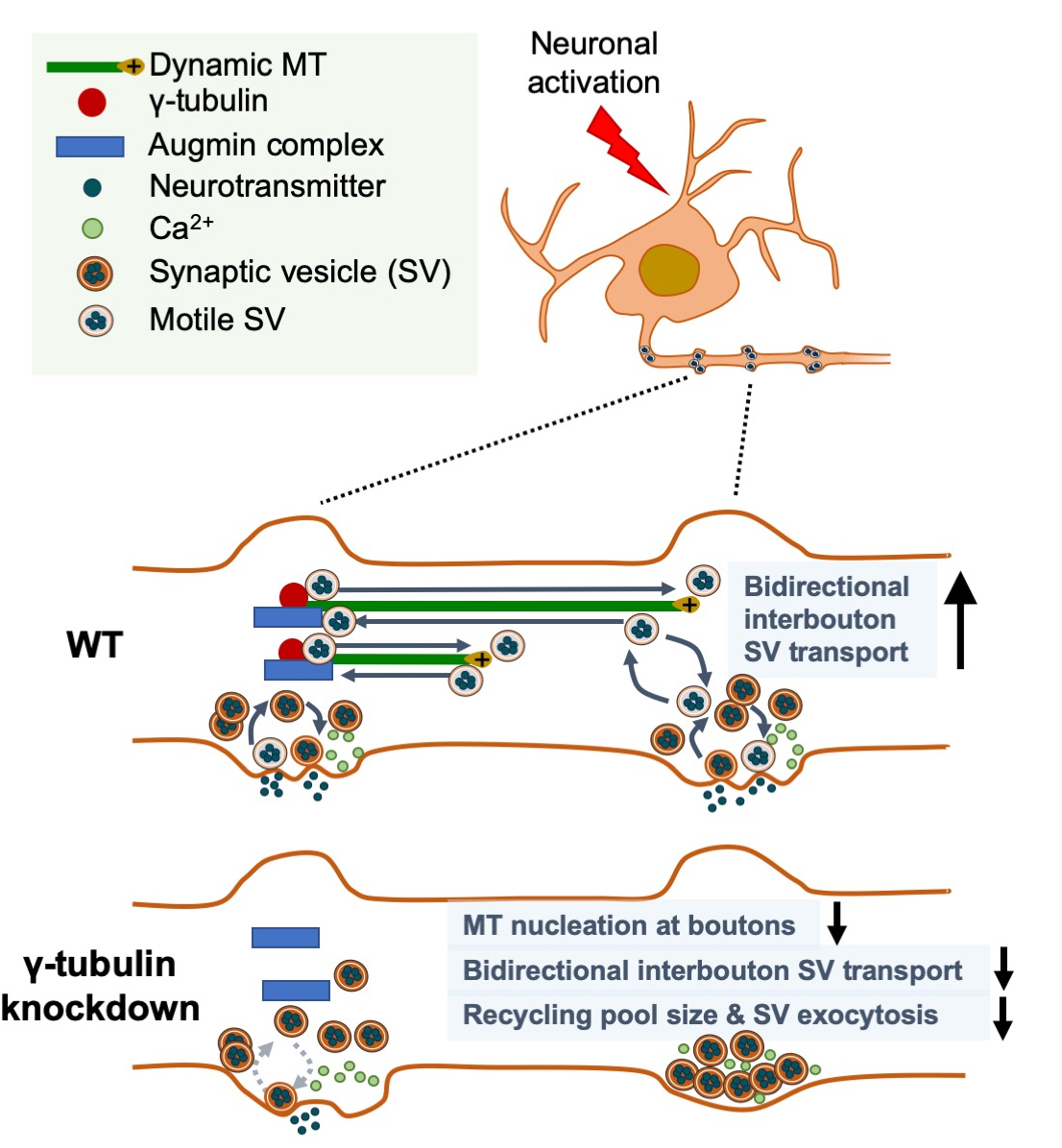
A new study by the laboratory of Dr. Francesca Bartolini, including first author Dr. Xiaoyi (Lily) Qu (now at Genentech), provides important insight into these questions with the surprising discovery that en passant presynaptic boutons of hippocampal neurons are hotspots for de novo nucleation of MTs, a function regulated by neuronal activity and rate-limiting for neurotransmission. Featured in the latest issue of Current Biology, with an accompanying editorial, they report that dynamic microtubules emerge at excitatory presynaptic boutons in vitro and ex vivo, as a result of restricted g-tubulin- and augmin-dependent de novo microtubule nucleation, with g-tubulin regulating the nucleation density, and augmin directing the uniform, plus-end directed growth towards the distal end of the axon. Importantly, Qu et al. found that de novo nucleation of dynamic microtubules is regulated by neuronal activity, required for bi-directional interbouton synaptic vesicle motility, and critical for synaptic vesicle exocytosis at sites of release.
These findings unveil a role for activity-dependent microtubule nucleation at en passant boutons in synaptic interbouton delivery that controls neurotransmission. Given the critical role that loss of a functional presynaptic machinery for MT nucleation may have for neurotransmission, it will be important to determine the in vivo significance of this selective MT nucleation, and whether dysregulation of this process can be associated with human neurological and neuropsychiatric illnesses caused by mutations in MT regulatory proteins residing presynaptically. Examples of such include tau in Alzheimer’s disease and other tauopathies, spastin in hereditary spastic paraplegias, and MAP1B and FMRP in Fragile X Syndrome.
Francesca Bartolini, PhD
Assistant Professor of Pathology & Cell Biology
fb2131@cumc.columbia.edu
Role of Tau Protein in Remodeling of Circadian Neuronal Circuits and Sleep
 |  |
|
| Mercedes Arnes, PhD | Ismael Santa-Maria Perez, PhD |
Circadian rhythms impose daily cycles in a wide variety of behaviors and physiological processes. Alzheimer’s disease (AD) and related tauopathies are associated with a decay of circadian rhythms and the disruption of sleep patterns and cognitive function, but the mechanisms underlaying these alterations remain unclear. Traditional approaches in neurodegeneration research have focused on understanding how pathology impinges on circadian function. However, recent evidence demonstrates that circadian and sleep-wake cycle disruption often occur early in the course of the disease and may even precede the development of cognitive symptoms. Since tau proteostasis is compromised in AD and related tauopathies, Dr. Ismael Santa-Maria and colleagues sought to understand the role tau protein plays in neuronal circadian biology and related behavior. Their findings were recently published in Frontiers in Aging Neuroscience.
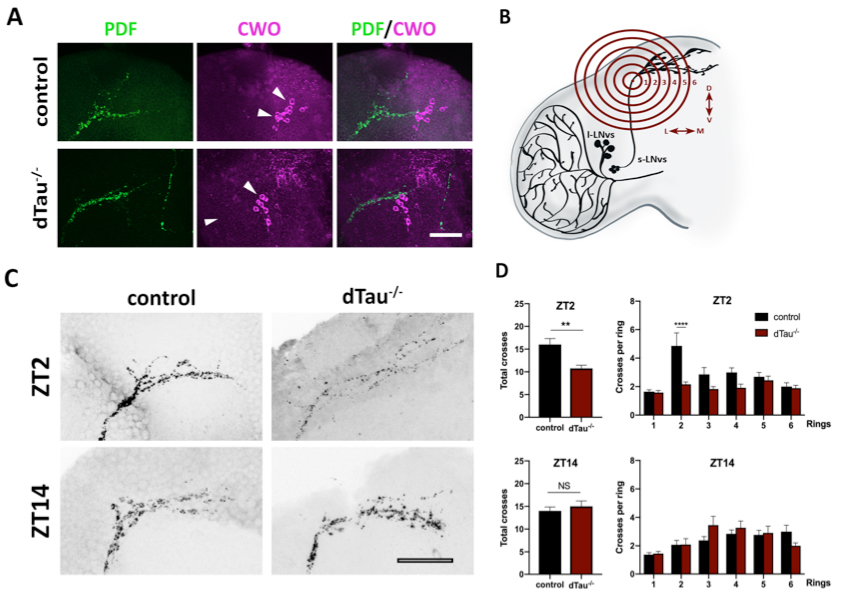 Figure 1. Effect of tau deficiency on daily rhythms of axonal structural remodeling. (A) Immunolabeling with anti-PDF and anti-CWO antibodies in LNv and DN neurons of adult brains from 7 day-old male flies. CWO immunostaining denotes the proper localization and morphology of DN neurons (arrow heads). Scale bar is 20 μm. (B) Scheme representing Sholl analysis in PDF-positive (black) LNv projections. Six concentric and evenly spaced (10 μm) rings (in red) are drawn. The centers of the rings are localized at the point where the first dorsal ramification starts in each hemisphere. (C) Representative confocal images of LNv neurons immunolabeled with anti-PDF antibody at ZT-2 and ZT-14, early morning and early evening, respectively. Panels show PDF-positive signal in LNv projections. Scale bar is 25 μm. (D) Plots representing Sholl analysis quantification at ZT2 and ZT14 for the corresponding genotypes. dTau–/– flies showed a significant reduction in the spreading across the concentric rings specifically at ZT-2, when compared to controls. No changes were found at ZT-14. Data represent mean and SEM analyzed by one-way ANOVA test, with ∗∗p < 0.01 and ****p < 0.0001 or NS if no statistical significance (n = 12–14 hemispheres).
|
Santa-Maria Lab picture. Mercedes Arnes, first author on the right Joshua Cho, another contributing author on the left.
Taken together, results by Arnes et al. suggest that loss of tau function participates in the regulation of circadian rhythms by modulating the correct operation and connectivity of core circadian networks and related behavior. The Santa-Maria lab will continue exploring mechanisms of tau regulation in clock neurons. Stay tuned!
Mercedes Arnes, PhD
Postdoctoral Research Scientist in the Department of Pathology and Cell Biology
ma3661@cumc.columbia.edu
Ismael Santa-Maria Perez, PhD
Assistant Professor of Pathology and Cell Biology
is2395@cumc.columbia.edu
 |  |  |
|||
| Marta Olah, PhD | Elizabeth M. Bradshaw, PhD | Philip L. De Jager, MD, PhD |
Sleep disruption may contribute to cognitive impairment and dementia in older adults. Sleep disruption has also been shown to result in gene expression changes characteristic of immune dysregulation, which in turn, might contribute to cognition-related disease processes, including Alzheimer’s disease (AD). Thus there is a possibility that sleep disruption may contribute to cognitive impairment through an immune mechanism. Microglia, the resident innate immune cells of the central nervous system, may play a key role in this. A role for microglia in AD has been implicated by genetic studies. Moreover, in rodents, chronic sleep restriction or deprivation can alter the immune signaling milieu in a way known to trigger changes in microglial function and can lead to morphologic microglial activation, and inhibiting this can improve cognition. However, data relating sleep, microglial biology, and cognition in humans are lacking—an important gap given differences in microglial biology between mice and humans.
Recently, a transcriptomic atlas of aged human microglia was established by Drs. Marta Olah, Elizabeth Bradshaw, and Philip L. De Jager from the Taub Institute and CTCN. In the present study, published recently in Science Advances, Olah and colleagues used this transcriptomic signature (coined the HuMi-Aged gene set) and the differentially expressed genes to disentangle the connection between microglia activation, aging, sleep fragmentation and cognitive decline. Working together with colleagues from multiple institutions, all datasets (actigraphy, cognitive assessment, cortical gene expression, AD pathology, microglia activation and microglia gene expression) originated from two prospective cohorts of aging and dementia at RUSH University: the Religious Orders Study and the Memory and Aging Project. In this cross-sectional study of older community-dwelling adults, they found that greater sleep fragmentation was associated with higher expression of genes characteristic of aged microglia and a greater density of morphologically activated microglia. As recently published in, the transcriptional changes were independent of chronological age, density of microglia, and dementia-related brain pathologies and were not completely accounted for by the increased density of morphologically activated microglia. Moreover, greater expression of genes characteristic of aged microglia was associated with worse cognition and partially accounted for the association between sleep fragmentation and worse cognition. These findings raise the possibility that microglial aging and activation may be a consequence of sleep fragmentation and may establish microglia as a link between sleep fragmentation and poor cognition in older adults.
Marta Olah, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain)
mo2738@cumc.columbia.edu
Elizabeth M. Bradshaw, PhD
Adler Assistant Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Institute for Genomic Medicine)
emb2280@cumc.columbia.edu
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu

