Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
July 2025
Early Proteasome Downregulation and Dysfunction Drive Proteostasis Failure in Alzheimer's Disease
The Role of Alpha-Synuclein in Synucleinopathy: Impact on Lipid Regulation at Mitochondria-ER
Associations Between Hearing Loss and Dementia in a Large Electronic Health Record System
APOE and Alzheimer's Disease and Related Dementias Risk Among 12,221 Hispanics/Latinos
A Human Brain Map of Mitochondrial Respiratory Capacity and Diversity
ANXA11 Biomolecular Condensates Facilitate Protein-Lipid Phase Coupling on Lysosomal Membranes
Axonal Transport of CHMP2b Is Regulated by Kinesin-Binding Protein and Disrupted by CHMP2bintron5
Sleep Genetics and Cognitive Changes over Time: The Moderating Effect of Age and the Role of Brain
Emerging Roles for Tubulin PTMs in Neuronal Function and Neurodegenerative Disease
Inflammatory Biomarkers Profiles and Cognition Among Older Adults
Synaptic and Cognitive Impairment Associated with L444P Heterozygous Glucocerebrosidase Mutation
Regulation of Synapse Density by Pumilio RNA-Binding Proteins
CD33 and SHP-1/PTPN6 Interaction in Alzheimer's Disease
Cellular Communities Reveal Trajectories of Brain Ageing and Alzheimer's Disease
Epigenetic and Genetic Risk of Alzheimer Disease from Autopsied Brains in two Ethnic Groups
Multi-Omic Analysis of Huntington's Disease Reveals a Compensatory Astrocyte State
Design and Methods of the Early Age-Related Hearing Loss Investigation Randomized Controlled Trial
Updated Safety Results From Phase 3 Lecanemab Study in Early Alzheimer's Disease
The Broken Alzheimer's Disease Genome
Rare Genetic Variation in Fibronectin 1 (FN1) Protects Against APOEε4 in Alzheimer's Disease
Cell Subtype-Specific Effects of Genetic Variation in the Alzheimer's Disease Brain
Diet, Pace of Biological Aging, and Risk of Dementia in the Framingham Heart Study
A Comparative Study of Structural Variant Calling in WGS from Alzheimer's Disease Families
Glucocorticoid Stress Hormones Stimulate Vesicle-Free Tau Secretion and Spreading in the Braint
The Effects of Insufficient Sleep and Adequate Sleep on Cognitive Function in Healthy Adults
ZCCHC17 Modulates Neuronal RNA Splicing and Supports Cognitive Resilience in Alzheimer's Disease
Effects of Lithium on Serum Brain-Derived Neurotrophic Factor in Alzheimer's Patients with Agitation
2023 Taub Institute Grants for Emerging Research (TIGER) Awardees!
Rie1 and Sgn1 Form an RNA-Binding Complex that Enforces the Meiotic Entry Cell Fate Decision
Memory and Language Cognitive Data Harmonization Across the United States and Mexico
Education as a Moderator of Help Seeking Behavior in Subjective Cognitive Decline
Multicellular Communities are Perturbed in the Aging Human Brain and Alzheimer's Disease
The Neuropathological Landscape of Hispanic and non-Hispanic White Decedents with Alzheimer Disease
The Early-Onset Alzheimer's Disease Whole-Genome Sequencing Project: Study Design and Methodology
Polygenic Risk Score Penetrance & Recurrence Risk in Familial Alzheimer Disease
High School Quality is Associated with Cognition 58 Years Later
Glucocorticoid-Driven Mitochondrial Damage Stimulates Tau Pathology
A Global View of the Genetic Basis of Alzheimer Disease
ARIA in Patients Treated with Lecanemab (BAN2401) in a Phase 2 Study in Early Alzheimer's Disease
Microglia Reactivity Entails Microtubule Remodeling from Acentrosomal to Centrosomal Arrays
Genuine Selective Caspase-2 Inhibition with new Irreversible Small Peptidomimetics
Cell Type-Specific Changes Identified by Single-Cell Transcriptomics in Alzheimer's Disease
Brain Aging Among Racially and Ethnically Diverse Middle-Aged and Older Adults
First Place: Neuroproteasome Localization and Dysfunction Modulate Pathology in Alzheimer's Disease
Clearance of an Amyloid-Like Translational Repressor is Governed by 14-3-3 Proteins
Diet Moderates the Effect of Resting State Functional Connectivity on Cognitive Function
Retromer Deficiency in Tauopathy Models Enhances the Truncation and Toxicity of Tau
Progranulin Mutations in Clinical and Neuropathological Alzheimer's Disease
Wolframin is a Novel Regulator of Tau Pathology and Neurodegeneration
Homotypic Fibrillization of TMEM106B Across Diverse Neurodegenerative Diseases
Correlation of Plasma and Neuroimaging Biomarkers in Alzheimer's Disease
Tubulin Tyrosination Regulates Synaptic Function and is Disrupted in Alzheimer's Disease
The Penalty of Stress - Epichaperomes Negatively Reshaping the Brain in Neurodegenerative Disorders
The Neuronal Retromer can Regulate Both Neuronal and Microglial Phenotypes of Alzheimer's Disease
Deep Learning Improves Utility of Tau PET in the Study of Alzheimer's Disease
Age of Onset of Huntington's Disease in Carriers of Reduced Penetrance Alleles
Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease
Midlife Vascular Factors and Prevalence of Mild Cognitive Impairment in Late-Life in Mexico
The Association Between Sex and Risk of Alzheimer's Disease in Adults with Down Syndrome
Marked Mild Cognitive Deficits in Humanized Mouse Model of Alzheimer's-Type Tau Pathology
Rapid ATF4 Depletion Resets Synaptic Responsiveness after cLTP
Polygenic Risk Score for Alzheimer's Disease in Caribbean Hispanics
Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia
The Microtubule Cytoskeleton at the Synapse & The Synaptic Life of Microtubules
Optimizing Subjective Cognitive Decline to Detect Early Cognitive Dysfunction
The AD Tau Core Spontaneously Self-Assembles and Recruits Full-Length Tau to Filaments
Olfactory Impairment is Related to Tau Pathology and Neuroinflammation in Alzheimer's Disease
Pathogenic Role of Delta 2 Tubulin in Bortezomib-Induced Peripheral Neuropathy
2: Phase Contrast-Derived Cerebral Blood Flow is Associated with Neurodegeneration and Cerebrovascular Injury in Older Adults
3: Accelerating Biomedical Discoveries in Brain Health Through Transformative Neuropathology of Aging and Neurodegeneration
 |  | |
| Richard Mayeux, MD, MSc | Mark Logue, PhD |
As part of a multi-institutional study led by researchers from Boston University, we recently explored how genetic risk for Alzheimer’s disease may differ across subgroups within African American populations. Using genome-wide association data from both the VA Million Veteran Program and the Alzheimer’s Disease Genetics Consortium, we stratified analyses by sex, age at onset, and APOE-ε4 status—factors that are often biologically meaningful but underexamined in combination, particularly in non-European cohorts.
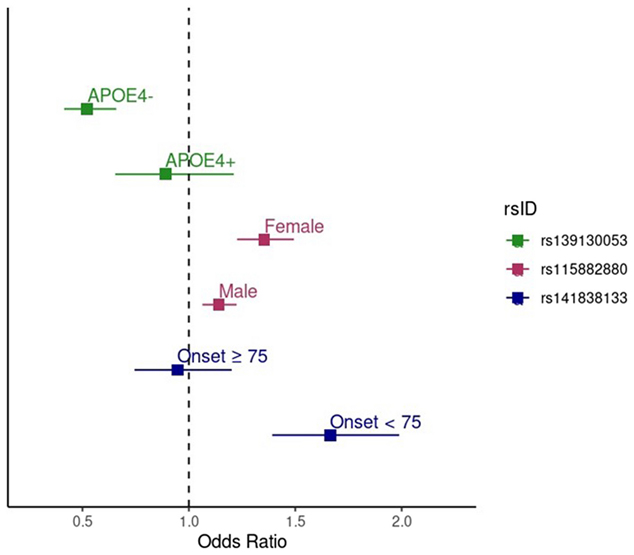
Figure 2. Forest plot showing the effect sizes (odds ratios) and 95% confidence intervals for the MVP/ADGC meta-analysis results for the genome-wide significant SNPs in both the stratum in which the association was identified and the opposite stratum. The green points show the effect of rs139130053 in APOE-ε4 negative individuals (upper green point) and APOE-ε4 positive individuals (lower green point). The pink points show the effect of rs115882880 in females (upper pink point) and males (lower pink point). The blue points show the effect of rs141838133 in individuals with onset age ≥ 75 years (upper blue point) and individuals with onset age < 75 years (lower blue point).
As recently reported in Alzheimer’s Research & Therapy, this approach uncovered three genome-wide significant loci outside of the APOE region: a variant near EPHA5 associated with earlier onset cases, a signal in GRIN3B (next to ABCA7) that was strongest in females, and a variant near TSPEAR in APOE-ε4 non-carriers. These genes are implicated in pathways related to synaptic plasticity, insulin signaling, and protein degradation—mechanisms that are increasingly recognized as relevant to Alzheimer’s disease pathophysiology.
These findings add to growing evidence that stratified genetic analysis—particularly in underrepresented populations—can reveal novel risk loci that may be missed in more generalized approaches. They underscore the importance of both diverse population representation and biologically informed subgroup analysis in dementia genetics. Together, these strategies offer a more nuanced view of Alzheimer’s disease risk and help illuminate the complexity of its genetic basis across different groups.
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Sergievsky Center and Taub Institute)
rpm2@cumc.columbia.edu
 |  |  | ||
| Adam M. Brickman, PhD | Jennifer J. Manly, PhD | Richard Mayeux, MD, MSc |
2222222 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
2222222 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
2222222 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  |  | ||
| Cláudio Gouveia Roque, PhD | Hemali Phatnani, PhD | Ulrich Hengst, PhD |
3333333 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
3333333 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
3333333 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
33333
Page and William Black Professor of Neurology (in Pathology and Cell Biology and Neuroscience)
sp30@cumc.columbia.edu
 |  | |
| James Noble, MD, MS | Justin Golub, MD, MS |
444444 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
444444 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
444444 Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum.
Hemali Phatnani, PhD
Assistant Professor of Neurological Sciences (in Neurology)
hp2286@cumc.columbia.edu

