Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
September 2022
2: Deep Learning of MRI Contrast Enhancement for Mapping Cerebral Blood Volume from Single-Modal Non-Contrast Scans of Aging and Alzheimer's Disease Brains
3: Socioeconomic Status, Biological Aging, and Memory in a Diverse National Sample of Older US Men and Women
 |
 |  | |
| Julie Parato, PhD | Francesca Bartolini, PhD | Leticia Peris, PhD |
Microtubules (MTs) support a range of neuronal functions, from intracellular transport to synaptic plasticity. MTs can develop a variety of post-translational modifications (PTMs), such as detyrosination and acetylation. These PTMs act as a regulatory code, affecting the binding of MT associated proteins and motor proteins, as well MT longevity. The tubulin detyrosination/re-tyrosination cycle is a key player in the maintenance of MT dynamics, as tyrosinated tubulin is associated with more dynamic MTs, while detyrosinated tubulin is linked to more stable, longer-lived MTs. We have previously demonstrated that decreased tubulin re-tyrosination is correlated to Alzheimerās disease (AD) progression, changes in MT dynamics, and widespread synapse loss. Although tubulin acetylation and detyrosination occur at different locations on the MT wall and are driven by different regulatory enzymes, both PTMs are frequently observed in the same sub-population of stable MTs. While MT acetylation has long received attention in the context of AD, however, the literature on this topic has been varied.
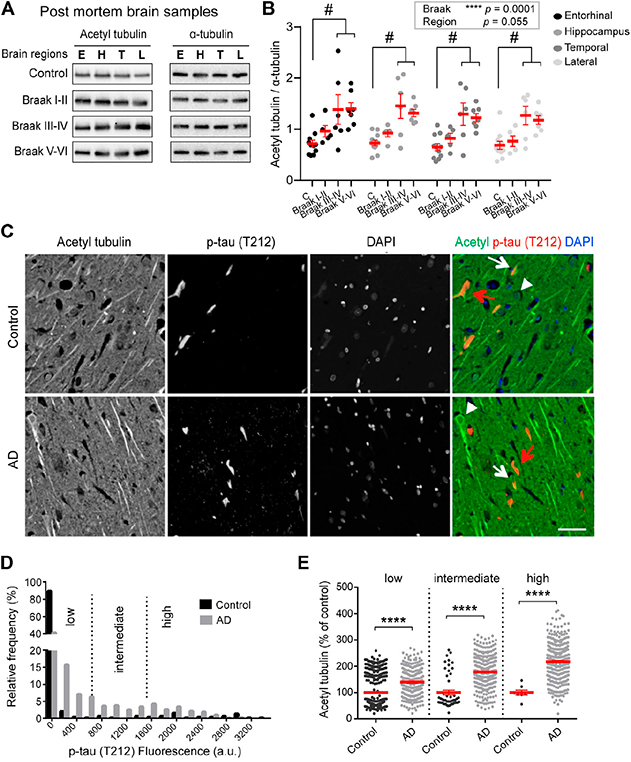
Figure 1. Increased acetylated Ī±-tubulin correlates with phospho-tau accumulation in AD. (A-B) Acetylated (acetyl) tubulin and total α-tubulin levels in brain homogenates from entorhinal cortex (E), hippocampus (H), temporal (T), and lateral prefrontal cortex (L) from control, early AD (Braak I-II), middle AD (Braak III-IV), and late AD (Braak V-VI) patients. (C-E) Dual immunostaining and analysis of acetylated Ī±-tubulin and T212-reactive phospho-tau, combined with nuclear staining with DAPI, was performed on sections of control (upper panel) and AD patient hippocampi (lower panel). Neurons with low (white arrowheads), intermediate (white arrows) or high (red arrows) levels of T212 immunofluorescence are shown.
With Dr. José Martínez-Hernández and Dr. Leticia Peris, we explored tubulin acetylation in AD by analyzing brain samples obtained after short post-mortem intervals. As recently report in Frontiers in Cell and Developmental Biology, in the AD brain samples, up-regulation of tubulin acetylation was already detectable at early AD stages and became statistically significant at middle and late stages. To determine whether the increase of tubulin acetylation was a hallmark of familial AD, we chose to evaluate hiPSC-derived human neurons harboring the AD London mutation, in collaboration with Taub faculty member Dr. Andrew Sproul. Consistent with the patient data, acetylated tubulin was significantly higher in the London mutation-carrying neurons than in isogenic controls.
Interestingly, we observed no correlation in the amounts of Ī±-TAT1 (tubulin acetylase) or HDAC6 (deacetylase) with the acetylation levels so we examined the functional crosstalk between acetylation and the detyrosination/re-tyrosination cycle of tubulin in healthy cultured neurons. We found that by increasing detyrosinated tubulin, we were able to enhance tubulin acetylation while by reducing detyrosinated tubulin, we were able lower acetylated tubulin levels. Based on this evidence we propose a model by which loss of tubulin re-tyrosination in AD drives an increase in MT longevity that also accounts for higher levels of tubulin acetylation. Whether this occurs because longer-lived detyrosinated MTs are an easier target for α-TAT1 in health and AD remains to be determined.
Julie Parato, PhD
Postdoctoral Research Scientist in Pathology and Cell Biology
jap2299@cumc.columbia.edu
Francesca Bartolini, PhD
Associate Professor of Pathology and Cell Biology
fb2131@cumc.columbia.edu
 |
 |  | |
| Frank Provenzano, PhD | Scott Small, MD | Jia Guo, PhD |
Gadolinium based contrast agents (GBCA) are administered frequently in the clinical setting to enhance brain MRI scans and can be useful to generate high resolution functional brain maps. These maps have been useful in isolating potential sites of metabolic dysfunction, however, there are rising concerns over the safety and invasiveness of the injection of such contrast agents. In a study led by Dr. Jia Guo (Psychiatry), together with Dr. Scott Small and other colleagues from Columbia, we sought to explore the feasibility of using a convolutional neural network (CNN) deep learning model, trained on the largest assembly of research quality gadolinium enhanced MRI, to determine the feasibility of using deep learning to enhance MRI with GBCA without injection of a contrast agent. Our aim was to determine if the resultant functional maps were able to replicate dysfunction previously found using injected agents.
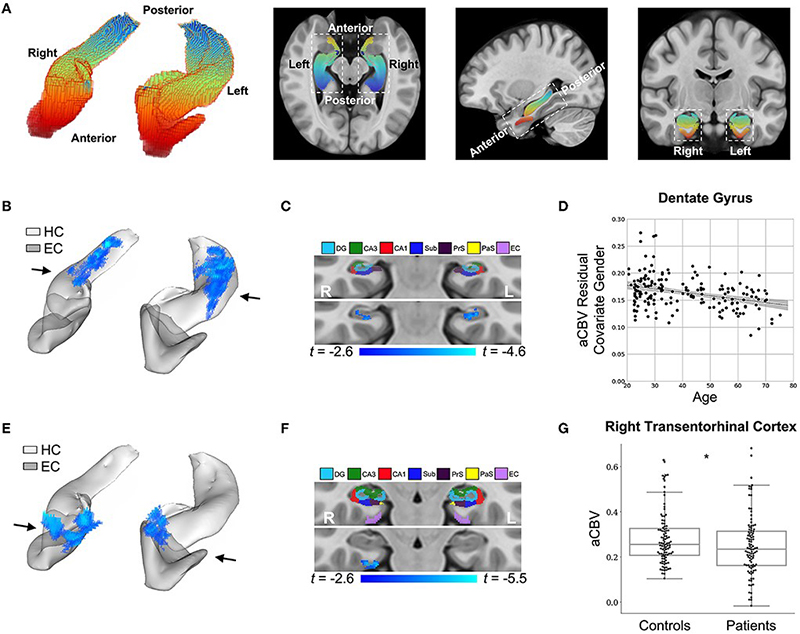
Figure 4: DeepContrast maps differential anatomical patterns of dysfunction in the hippocampal formation.
As recently reported in Frontiers in Aging Neuroscience, models were trained to substitute GBCA in MRI for both mice and humans, using a train validation test split of 326/93/179 for human studies. Studies were performed to evaluate GBCAās added discrimination in Alzheimerās disease classification. Specifically, using synthetic GBCA generated in a test-data set, changes to normal aging were discovered within the dentate gyrus, as we previously reported, and changes in the entorhinal cortex were discovered in AD. In a separate dataset, models were trained to examine the contribution of synthetic cerebral blood volumes maps from structural neuroimaging; we found that, by adding those images, the receiver operating characteristic area under the curve improved from .905 to .942.
Overall, results of this study by Liu, Zhu, et al. show that it may be possible to derive functional neuroimaging measures from structural neuroimaging using only deep learning and a specially trained dataset. Further, it may be possible to not only use structural neuroimaging to generate contrast enhanced images without an injection, but that those images may be useful in identifying āfunctional lesionsā in those individuals.
Frank Provenzano, PhD
Assistant Professor of Neurological Sciences (in Neurology and in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
fap2005@cumc.columbia.edu
 |  | |
| Justina F. Avila-Rieger, PhD | Jennifer J. Manly, PhD |
Exposure to socioeconomic (SES) disadvantage is associated with early-onset cognitive aging. Biological aging, the progressive loss of system integrity that occurs as we age, is proposed as a modiļ¬able process mediating this health inequality. Until recently, we have not had a way of comprehensively measuring these biological processes. DNA-methylation (DNAm) biomarkers that quantify molecular alterations that occur with aging are emerging as a powerful approach for understanding how social exposures, such as SES, get āunder the skinā to shape health in aging. Few studies have described how these biomarkers relate to the process of cognitive aging. It is also unclear whether these biomarkers, that were developed in primarily White people, can equivalently quantify aging processes affecting cognition in Black and Latinx individuals.
Together with senior author Dr. Jennifer J. Manly, we examined whether accelerated biological aging explains socioeconomic disparities in cognitive aging in 3,997 mid-to late-life adult participants of the U.S. Health and Retirement Study (HRS) DNA-methylation sub-study. As recently reported in Neurology, we found that among White participants, accelerated biological aging partially explained the socioeconomic gradient in memory trajectories. Biological aging measures were inconsistently associated with memory trajectories and SES among Black and Latinx participants. Our findings suggest that these 3 DNAm measures have limited utility in quantifying biological aging pathways that link SES to cognition among Black and Latinx women and men.
Racially patterned lifecourse social experiences that inļ¬uence cognitive trajectories may shape epigenetic processes diļ¬erently across racial and ethnic groups, and such proļ¬les may not be adequately represented with these current DNAm measures. Racism is associated with exposure to a myriad of acute and chronic stressors that accumulate over the lifecourse and accelerates physiologic deterioration. More research is needed to determine whether the cumulative stress exposure experienced by Black and Latinx people is associated with distinct epigenetic pathways (e.g., associated with diļ¬erent CpG sites or diļ¬erentially methylation regions).
Justina F. Avila-Rieger, PhD
Associate Research Scientist
Taub Institute for Research on Alzheimer's Disease and the Aging Brain
jfa2125@cumc.columbia.edu
Jennifer J. Manly, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute and the Gertrude H. Sergievsky Center)
jjm71@cumc.columbia.edu

