Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
September 2021
2: Epigenomic Features Related to Microglia are Associated with Attenuated Effect of APOE Īµ4 on Alzheimer's Disease Risk in Humans
3: Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease
 |  | |
| Yiyi Ma, MD, PhD | Philip L. De Jager, MD, PhD |
The apolipoprotein E (APOE) Īµ4 haplotype contributes the greatest common genetic risk for Alzheimer's disease (AD). However, not all Īµ4 carriers that survive to advanced age develop AD, suggesting that factors attenuating the risk of Īµ4 on AD may exist. Identifying such an APOE Īµ4 attenuator could give us novel and meaningful insights into the development of new, targeted treatments for AD and enable personalized treatment of Īµ4 carriers.
Therefore, the main focus of the current study from our laboratory was to identify those modifiable CpG dinucleotides that may act as an APOE Īµ4 attenuator. Guided by the top Īµ4-attenuating signals from methylome-wide association analyses (N=572, Īµ4+ and Īµ4-) of neurofibrillary tangles and neuritic plaques, we conducted a meta-analysis for pathological AD within the Īµ4+ subgroups (N=235) across four independent collections of brains. Cortical RNA-seq and microglial morphology measurements were used in functional analyses. Three out of the four significant CpG sites were captured by one principle component (PC1), which interacts with Īµ4 on AD, and is associated with expressions of innate immune genes and activated microglia. In Īµ4 carriers, reduction in each unit of PC1 attenuated the odds of AD by 59% (OR=2.41, 95%CI=[1.64, 3.52], P=7.40Ć10ā6).
Overall, we report in Alzheimerās & Dementia that the deleterious effect of the strongest genetic risk (APOE Īµ4) for AD may be attenuated by an epigenomic factorāepigenomic factor of activated microglia (EFAM)ā that could work, at least in part, through alterations in the relative proportion of activated microglia (as defined morphologically) and, subsequently, on the accumulation of tau pathology. This suggests that reduction of microglial activity could be a particularly effective intervention in APOE Īµ4+ individuals who already have evidence of amyloid pathology on imaging or cerebrospinal fluid. Further, it yields hypotheses that now need to be tested mechanistically to validate our results and support the proposed sequence of events in which activation of microglia has a strong effect on the accumulation of tau pathology in APOE Īµ4+ individuals.
Yiyi Ma, MD, PhD
Instructor in Neurological Sciences (in Neurology) at CUIMC
ym2666@cumc.columbia.edu
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in the Taub Institute)
pld2115@cumc.columbia.edu
Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease
 |  | |
| Maria I. Avrutsky, PhD | Carol M. Troy, MD, PhD |
Caspases are a family of cysteine-aspartic proteases that mediate programmed cell death and inflammation by selectively cleaving substrate proteins. First identified as an apoptotic activation factor in 1997, caspase-9 is now nearly synonymous with its role as an initiator of intrinsic apoptosis. Our group has implicated caspase-9 signaling in neuronal cell death following ischemic stroke and growth factor deprivation. Recently, we discovered a novel, nonapoptotic function of caspase-9 in ischemic endothelial cells, which contributes to vascular leakage and vision loss in a murine model of retinal vein occlusion. We demonstrated profound retinal protection by pharmacologically targeting caspase-9, supporting potential clinical development of a caspase-9 inhibitor for the treatment of ischemic retinal pathologies.
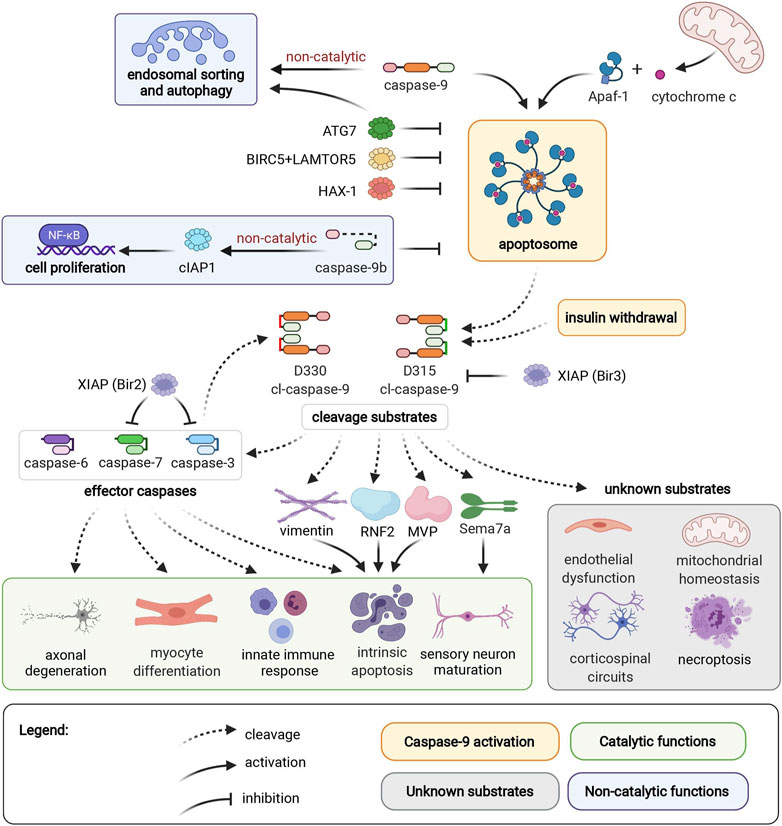
Figure 1. Caspase-9 activation, cleavage substrates, and cellular functions. See full figure. Created with BioRender.com
To follow up on these findings, we set out to explore the landscape of caspase-9 involvement in human health and disease. By integrating the recent advances in understanding caspase-9 cellular roles with a survey of its involvement in human pathologies, we assembled a comprehensive framework linking experimental studies with clinical literature on caspase-9 involvement in human disease.
In the current study in Frontiers in Pharmacology, we examine the expanded roles of caspase-9 beyond its traditional apoptotic functions. Caspase-9 plays distinct roles in cellular differentiation/maturation, innate immunity, mitochondrial homeostasis, and autophagy. Some functions depend on caspase-9 protease activity, and others, such as regulation of endosomal sorting, autophagy, and cell proliferation, are mediated by non-catalytic interaction between caspase-9 and various cellular partners.
From a clinical standpoint, both loss-of-function and excessive activation of caspase-9 can lead to devastating health outcomes. Genetic and histological evidence links caspase-9 with acute and chronic neurodegeneration, retinal neuropathy, slow-channel myasthenic syndrome, lumbar disc disease, cardiomyopathies, atherosclerosis, and autoimmune disease. We examine functional genetic studies on caspase-9, and discuss the implications for targeting caspase-9 as a therapeutic intervention in each indication. Our review evaluates the strength of evidence linking caspase-9 with each indication, and presents opportunities for future investigations to examine the consequences of caspase activity in human disease.
Maria I. Avrutsky, PhD
Scientist (Biology), Clover Therapeutics
mia2120@caa.columbia.edu
Carol M. Troy, MD, PhD
Professor of Pathology and Cell Biology and Neurology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain) at CUIMC
cmt2@cumc.columbia.edu

