Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
December 2022
2: RNA Methyltransferase NSun2 Deficiency Promotes Neurodegeneration through Epitranscriptomic Regulation of Tau Phosphorylation
3: Cell Type-Specific Changes Identified by Single-Cell Transcriptomics in Alzheimer's Disease
4: Brain Aging Among Racially and Ethnically Diverse Middle-Aged and Older Adults
5: Association of Subjective Cognitive Decline With Progression to Dementia in a Cognitively Unimpaired Multiracial Community Sample
 |
 |
| Kyung Min Chung, PhD | Ulrich Hengst, PhD |
Cells have developed several mechanisms to react to stress elicited by extrinsic cues or by disturbances of protein synthesis and secretion. Ideally, these pathways allow cells to reestablish homeostatic conditions and to survive. Only if the stress becomes insurmountable or prolonged, does stress signaling lead to degeneration and cell death. Cellular stress signaling has primarily been studied as a cell autonomous effect, i.e., how does an individual cell react to a stressor. However, especially for highly connected cells like neurons, the question of whether and how neurons can communicate stress to other neurons is intuitively important but only poorly understood.
|
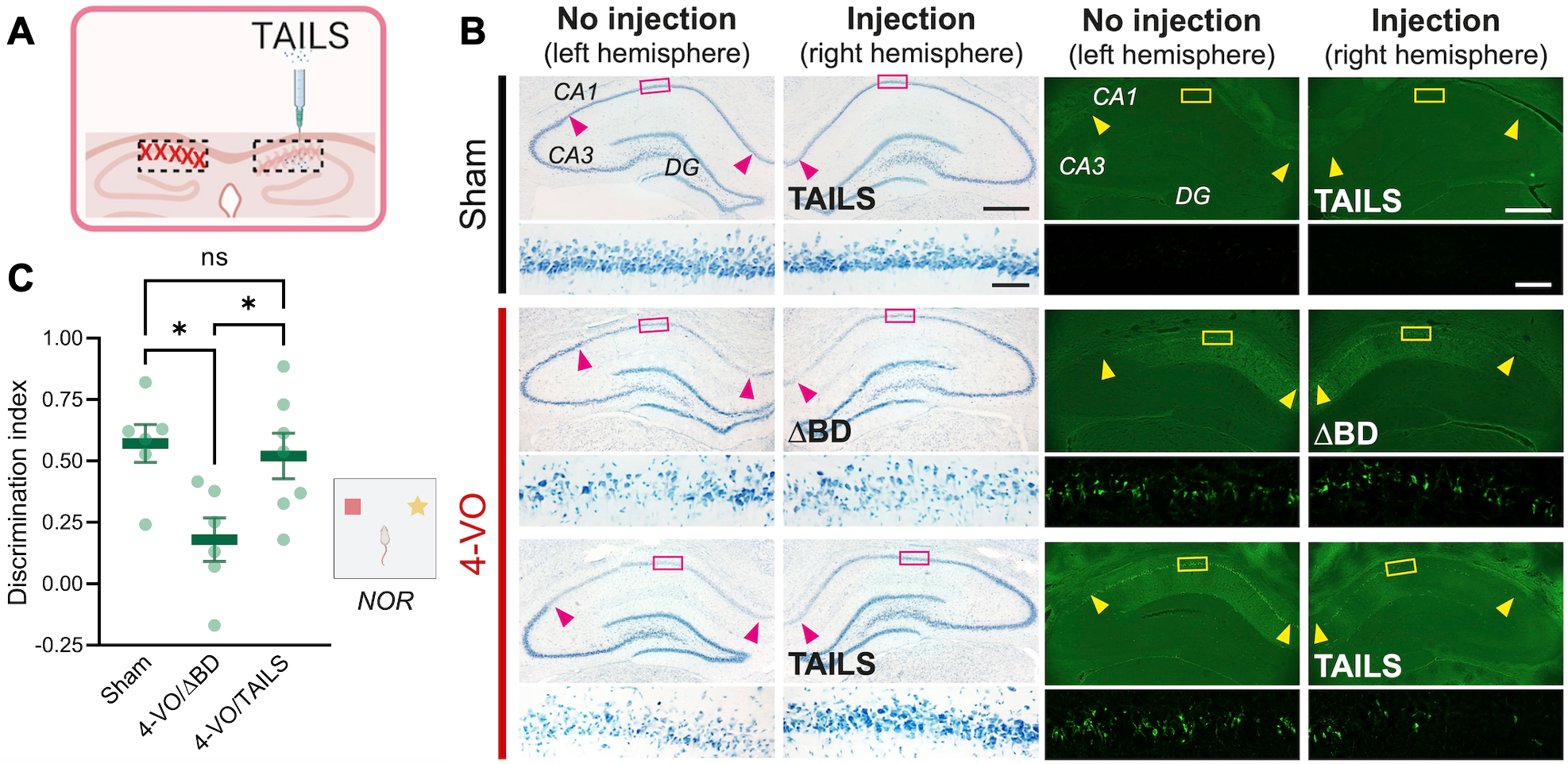
Figure 1. Administration of TAILS rescues 4-VO pathology (A) Scheme of TAILS injection into the hippocampus. (B) Histology analysis of CA1 hippocampal neurons in animals subjected for 4-VO or sham operation, administered with TAILS or ΔBD (100 ng μl-1; 3 μl per hemisphere) unilateral intrahippocampal injection. Brain sections stained with Toluidine Blue for living cells (left ) or Fluoro-Jade for degenerating cells (right). Scale bars, 500 μm and 50 μm (magnified). (C) Behavioral assessment of non-spatial memory by novel object recognition task in animals subjected for 4-VO or sham operation, administered with TAILS or the inactive ΔBD (100 ng μl-1; 3 μl per hemisphere). Discrimination index defined as [(novel object investigation time - familiar object investigation time)/(total investigation time)*100]. One-way ANOVA, Tukey’s multiple comparisons test. *, P<0.05.
|
In a study published in Cell Reports, we discovered and characterized a cell non-autonomous branch of cell stress signaling. In a collaboration with Dr. Jee-Yeon Hwang’s group at Creighton University, Omaha, NE, and Dr. Markus Siegelin’s group in the Department of Pathology & Cell Biology at CUIMC, lead author Kyung Min Chung from the Hengst laboratory in the Taub Institute discovered that in response to various stressors, including oxidative stress, neurons secrete a protein we named TAILS. TAILS potentiates SHH signaling in recipient cells, leading to increased mitochondrial function, and enhanced antioxidant capabilities. In short, TAILS acts as a secreted alarm signal, with which neurons under stress inform other neurons to up their defenses. To rigorously test the proposed mechanism in vivo, we chose a rodent model for global ischemia or stroke. The 4-vesicle occlusion (4-VO) model reliably leads to the death of pyramidal CA1 neurons in the hippocampus and causes cognitive impairment. Remarkably, administration of recombinant TAILS at the time of the procedure completely abolished the loss of CA1 neurons. Moreover, the TAILS-treated animals did not show any signs of hippocampus-dependent behavioral deficits, demonstrating that TAILS not only rescued the survival but also the function of CA1 neurons.
Importantly, we discovered that TAILS levels in the brain are upregulated in response to acute (stroke) and chronic (Alzheimer’s disease) neurodegenerative conditions and demonstrate that its administration boosts neuronal resilience in an ischemia model. Together, our data raise the exiting possibility of using TAILS therapeutically and potentially as a biomarker for the neuronal stress.
Ulrich Hengst, PhD
Associate Professor of Pathology & Cell Biology (in the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain
uh2112@cumc.columbia.edu
 |
 |  | |
| Yoon A. Kim, PharmD, PhD | Gunnar Hargus, MD, PhD | Ismael Santa-Maria Perez, PhD |
MicroRNAs are part of a vital regulatory mechanism that prevents the deposition of tau protein. Several studies have reported miR-125b is significantly upregulated in the frontal cortex and hippocampus in Alzheimer’s disease (AD) brains. MiR-125b upregulation induces tau hyperphosphorylation and cell death by targeting DUSP6 and PPP1CA phosphatases and activating CDK5 and p44/42-MAPK signaling in neurons. However, the molecular mechanisms governing how microRNA expression levels are dysregulated during the disease process are poorly understood.
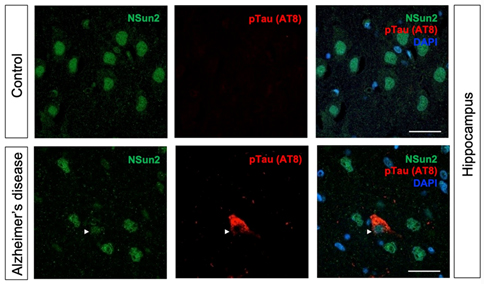
Together with Taub colleagues Drs. Caghan Kizil, Andrew Teich, Francesca Bartolini, Andrew Sproul, and in collaboration with Dr. Schahram Akbarian’s team in Icahn School of Medicine at Mount Sinai, we recently published a study in Acta Neuropathologica that shows how deficiency of RNA methyltransferase NSun2 in AD brains can lead to the buildup of toxic hyperphosphorylated forms of Tau and neurodegeneration. Using in vivo mouse and Drosophila models, human postmortem brain samples, and human iPSC-derived neurons, we found that decreased levels of neuroprotective NSun2 promotes epitranscriptomic alterations in miR-125b and tau hyperphosphorylation.
Our investigations further demonstrated that neuronal NSun2 levels decrease in response to amyloid-beta oligomers (AβO). Notably, AβO-induced tau phosphorylation and cell toxicity in human neurons could be rescued by overexpression of NSun2. Altogether, these results indicate that neuronal NSun2 deficiency promotes dysregulation of miR-125b and tau phosphorylation in AD and highlights a novel avenue for therapeutic targeting.
Ismael Santa-Maria Perez, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
is2395@cumc.columbia.edu
Gunnar Hargus, MD, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
gh2374@cumc.columbia.edu
Cell Type-Specific Changes Identified by Single-Cell Transcriptomics in Alzheimer's Disease
 |
 |
| Tain Luquez, PhD | Vilas Menon, PhD |
Alzheimer’s Disease (AD), the most common cause of dementia in elderly individuals, is a progressive neurodegenerative disease ultimately resulting in the loss of neurons and associated circuitry in the brain. In addition to this neuronal loss, genetics studies have implicated a range of non-neuronal cell types that are also likely affected in the disease. A new frontier in the study of AD has been the application of single-cell transcriptomics to characterize cell type-specific changes in human brain tissue. Indeed, over the past three years, several studies have profiled tissue from donors with and without diagnoses of AD, using cutting-edge single-cell approaches. These studies have highlighted key changes in cellular composition and molecular signatures associated with AD pathology and, in some cases, cognitive impairment. However, these studies have focused on different cell types and cohorts, making it difficult to assess which cell type changes are reproducibly found.
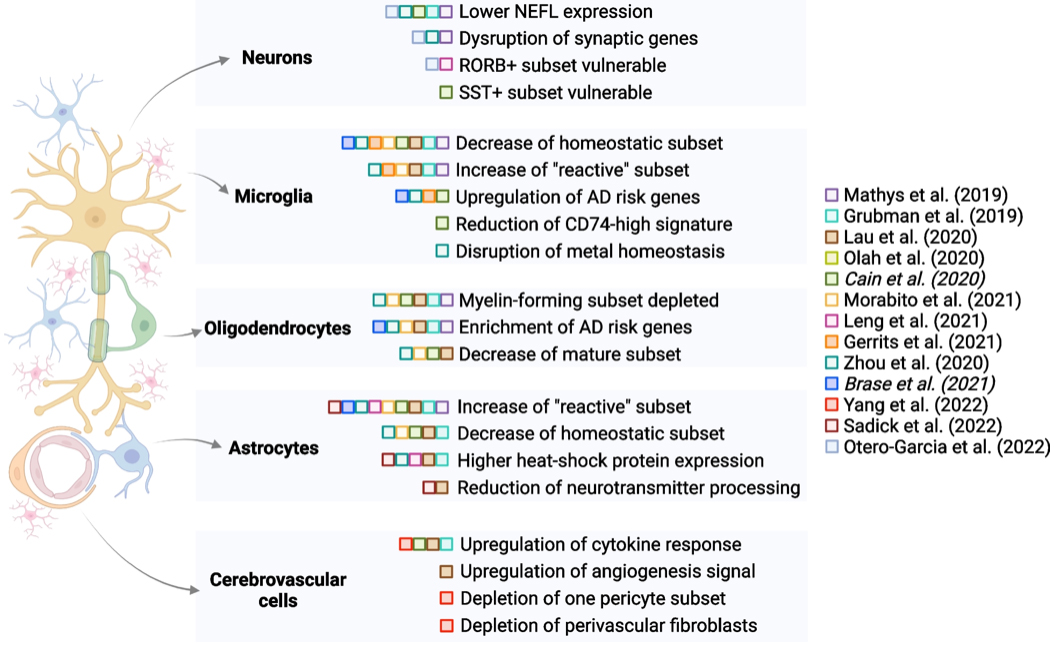
Figure 3. Schematic summary of selected findings from multiple sc/snRNA-seq studies, organized by cell type and publication date (italics indicate the Biorxiv versions of manuscripts). Studies have identified differences in all major cell types in brain tissue from healthy donors versus donors with AD diagnoses. Some of these differences are highlighted only in a subset of studies, suggesting the need for further exploration of reproducibility and consistency of findings. This figure was created using the BioRender package.
In the current study, we sought to synthesize findings from 13 studies (including work from our own lab in conjunction with Phil De Jager’s group in the Taub Institute); our goal was to identify which signatures are repeatedly found across studies and are likely to be the most robust cell type-specific changes observed in AD brain tissue. As reported in our recent review paper in Genome Medicine, we found that decreases in homeostatic microglial and astrocyte signatures, along with a reduction in myelin-forming oligodendrocytes, are noted in multiple studies, pointing to the involvement of these glial cell types in AD. Based on these compiled findings, we also touch upon new horizons in the field, particularly advancements in high-resolution spatial interrogation of tissue and multi-modal profiling at the single-cell level. Given rapid advancements in the field, we highlight how these new methods are likely to further advance mechanistic and target-prioritization studies on AD.
Vilas Menon, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute)
vm2545@cumc.columbia.edu
Brain Aging Among Racially and Ethnically Diverse Middle-Aged and Older Adults
 |
 |  | |
| Indira Turney, PhD | Jennifer J. Manly, PhD | Adam M. Brickman, PhD |
The weathering hypothesis suggests that repeated exposure to stress, suboptimal environments, and social disadvantage contributes to accelerated wear and tear on the body, leading to a faster rate of biological aging in Black and Latinx people. This hypothesis provides a framework to understand differential health outcomes across race and ethnicity groups across the life span, including differential brain aging and differential neurodegenerative disease.
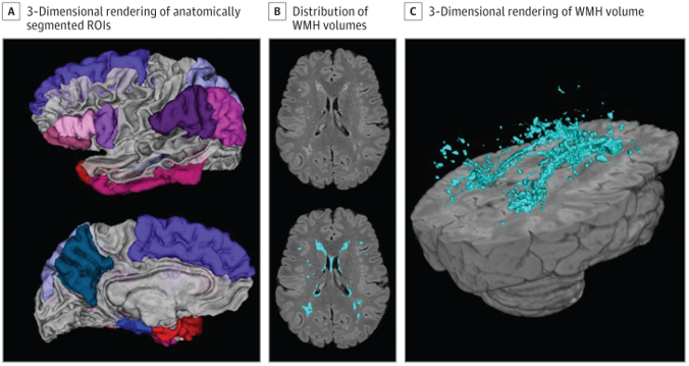
Figure 1. Neuroimaging Measures. A 3-dimensional rendering of the anatomically segmented regions of interest included in the Alzheimer disease signature composite (A). An axial slice displaying distribution of white matter hyperintensity (WMH) volumes unlabeled (B, top) with in-house developed software and WMH labeled (B, bottom). A 3-dimensional rendering of the labeled WMH volume (C). ROI indicates region of interest.
Previous studies by my group, Dr. Jennifer J. Manly, and others have suggested that minoritized racial and ethnic groups experience worse brain health in late life. It remains unclear, however, whether these disparities are apparent in midlife. In the current study, led by postdoctoral fellow and first author Dr. Indira Turney, we examined cortical thickness and white matter hyperintensity volume in midlife and late life across race and ethnicity groups. Magnetic resonance imaging (MRI) and neuropsychological testing from 1467 participants from the community-based WHICAP and Offspring studies were assessed. Data from both community-based studies provided a unique opportunity to examine these brain aging markers in diverse participants, across a wide age range (age 25-98 years), who also have health status, economic, and educational backgrounds that are representative of their communities.
As recently reported in JAMA Neurology, we found that racial and ethnic disparities in cerebrovascular disease occurred in both midlife and late life, while disparities in cortical thickness only occurred in late life. Overall, comparable with other reports, Black-White disparities were larger than Latinx-White disparities for both measures, while Black-Latinx disparities were minimal. Lastly, brain aging (i.e., the association of age with cortical thickness and WMH volume) was greater in late life than midlife for Latinx and White participants but not Black participants, who exhibited a similar magnitude of brain aging in midlife and late life. In other words, among Latinx and White participants, there was an inflection in age slopes for both measures from midlife to late life, while there was no difference or inflection in age slopes between midlife and late life Black participants, suggesting accelerated brain aging in middle-age Black individuals.
Adam M. Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute, and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  | |
| Silvia Chapman, PhD | Stephanie Cosentino, PhD |
Subjective cognitive decline (SCD), the subjective perception of decline in one’s cognition before such impairment is evident in traditional neuropsychological assessments, has emerged as a potential marker for a prodromal stage of dementia. Unfortunately, most studies examining SCD have been conducted in non-Latinx White samples and commonly exclude groups of individuals shown to be most vulnerable to dementia.
In the current study, our group, together with colleagues from Taub and Neurology, sought to more rigorously examine the utility of SCD as a predictor of dementia in diverse cohorts. Led by postdoctoral fellow and first author Dr. Silvia Chapman, we examined the predictive utility of SCD on progression to dementia in a cohort of 1,267 non-Latinx Black, 1,713 Latinxs and non-Latinx, and 1,063 Non-Latinx White older adults from the Washington Heights–Inwood Columbia Aging Project (WHICAP). As recently reported in Neurology, overall results of our study support SCD as a risk state for dementia in the full sample, and in Latinx and non-Latinx Black individuals in particular. Our findings highlight the importance of carefully evaluating any memory concerns raised by older adults during routine visits and underscore the potential utility of screening older adults for SCD.
Stephanie Cosentino, PhD
Associate Professor of Neuropsychology (in Neurology, the Taub Institute, and the Gertrude H. Sergievsky Center)
sc2460@cumc.columbia.edu