Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2022
2: Pyramidal Tract Neurons Drive Amplification of Excitatory Inputs to Striatum Through Cholinergic Interneurons
3: Associations Between Neuropsychiatric Symptoms and Neuropathological Diagnoses of Alzheimer Disease and Related Dementias
4: Longitudinal Associations Between Racial Discrimination and Hippocampal and White Matter Hyperintensity Volumes Among Older Black Adults
5: The Penalty of Stress - Epichaperomes Negatively Reshaping the Brain in Neurodegenerative Disorders
Tubulin Tyrosination Regulates Synaptic Function and is Disrupted in Alzheimer's Disease
 |
 |  | |
| Julie Parato, PhD | Xiaoyi (Lily) Qu, PhD | Francesca Bartolini, PhD |
Microtubules play fundamental roles in the maintenance of neuronal processes. Research over the past two decades has revealed that a pool of highly dynamic microtubules also critically regulates synaptic structure and function within both pre- and postsynaptic compartments (Parato el., al 2021; Waites et al., 2021). Dynamic microtubules provide the paths for bidirectional transport of synaptic vesicles between presynaptic terminals, a rate-limiting step in exocytosis at sites of release. In dendritic spines, while the core cytoskeletal structure consists of actin filaments, dynamic microtubules originating in the dendritic shaft sporadically enter the spine head and directly impinge on the regulation of spine composition and morphology. Despite this compelling evidence, however, whether perturbation of dynamic microtubules in neurons can cause synaptic pathology had remained unexplored.
Microtubule dynamics rely on the intrinsic capacity of microtubules to alternate phases of polymerization and depolymerization. Various cellular factors have been shown to modulate microtubule dynamics including various post-translational modifications of tubulin. One prominent modification is the reversible removal of the C-terminal tyrosine residue of α-tubulin subunits, which is exposed at the external surface of microtubules. When detyrosinated microtubules depolymerize, the tyrosine is rapidly restored on disassembled α-tubulin by the enzyme tubulin tyrosine ligase or TTL, thereby replenishing the soluble tubulin pool with full-length subunits that are then available for renewed polymerization. Detyrosinated microtubules can be further processed by cytosolic carboxypeptidases to generate Δ2 tubulin through the sequential cleavage of the final 2 amino acids. ∆2 tubulin cannot be re-tyrosinated by TTL and is thus permanently removed from the tyrosination/detyrosination cycle. It follows that while newly made dynamic microtubules are mainly composed of tyrosinated tubulin, long-lived microtubules contain detyrosinated and ∆2 tubulins, suggesting that the tubulin tyrosination/detyrosination cycle is a key player in the regulation of microtubule dynamics.
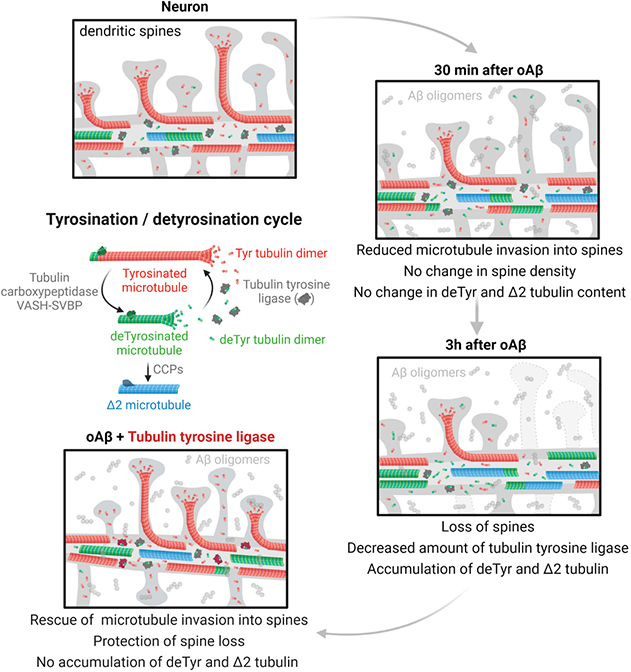 Graphical Abstract. Tyrosinated tubulin dimers polymerize into dynamic tyrosinated microtubules (red). Tubulin carboxypeptidases (VASH-SVBP) detyrosinate long-lived
microtubules (green). After depolymerization, tubulin tyrosine ligase (in grey) retyrosinates tubulin dimers. Very stable detyrosinated microtubules are substrate of cytosolic carboxypeptidases (CCPs) to form Δ2 microtubules (blue) that exits the tyrosination/detyrosination cycle. In mature neurons from control patients (or wild-type mice), tyrosinated microtubules form a shell at the outer part of the dendrite while detyrosinated and Δ2 microtubules localize to the inner part. Some dynamic microtubules from the dendrite transiently invade dendritic spines. In neuronal models of Alzheimer’s disease, Aβ oligomers exposure have a sequential effect on microtubule behavior and dendritic spine retraction: short time incubation with Aβ oligomers induces a decrease in TTL content, an accumulation of detyrosinated and Δ2 microtubules, a decrease in the frequency of microtubule invasion into spines with no change in dendritic spine density; longer incubation accentuates this phenotype and induces spine retraction. Ectopically controlled TTL expression restores tyrosinated, detyrosinated and Δ2 tubulin balance, microtubule invasion into the spines and dendritic spine density.
|
In this new study published in Brain and featured on CUIMC Newsroom, we hypothesized that loss of tubulin retyrosination induces synaptic dysfunction by reducing dynamic microtubules in spines. Indeed, we found that in the hippocampus of TTL hemizygous mice (TTL+/-), reduced levels of TTL led to significant changes in the tyrosinated/detyrosinated tubulin ratio and produced defects in synaptic transmission and plasticity that were associated with a loss of excitatory synapses. We examined whether TTL depletion was a feature of neurodegenerative disease and found that TTL expression was reduced in both sporadic and familial Alzheimer disease, and that abnormally high levels of detyrosinated and ∆2 tubulins accumulated in brain samples of Alzheimer’s disease patients. We explored whether tubulin retyrosination had a protective effect against the loss of synapses induced by oligomeric preparations of synthetic Aβ1-42 and found that while microtubule entry into spines protected neurons from spine pruning, acute Aβ exposure decreased the fraction of spines invaded by microtubules prior to spine loss. Remarkably, TTL expression inhibited both spine loss and the decrease in the fraction of spines invaded by microtubules, underscoring a role for retyrosinated tubulin in protecting synapses by driving dynamic microtubules into spines.
Our data unveil a role for the tyrosination/detyrosination tubulin cycle in regulating cognitive parameters such as dendritic spine density, synaptic plasticity and memory. They also provide compelling evidence for dysfunction of the cycle in Alzheimer’s disease and suggest that regulation of a-tubulin retyrosination may be critical for shielding synapses against Aβ-induced synaptic injury by promoting invasion of dynamic microtubules into spines.
Francesca Bartolini, PhD
Associate Professor of Pathology and Cell Biology
fb2131@cumc.columbia.edu

| 
|
Inbal Israely, PhD | Rui Costa, DVM, PhD |
Corticostriatal connections are central for many motor and cognitive processes. These cortical inputs to the striatum are plastic and are important for reinforcement and motor learning. Together with postdoctoral fellow and first author Dr. Nicolas Morgenstern and Master’s Student Ana Filipa Isidro (not pictured), we sought to address the differences in the signals that intratelencephalic (IT) and pyramidal tract (PT) cortical neurons convey to the spiny projection neurons in the striatum. For example, animals learning a motor task undergo changes at these connections. While the corticostriatal pathway was classically assumed to be homogeneous, there are different pathways between the cortex and striatum. We wanted to know more about the specific connectivity patterns of IT and PT neurons and their roles in this circuit.
We used transgenic mouse lines expressing ChR2 in each of these cortical populations independently. ChR2 allowed us to control axonal spiking with blue light. Thus, we could specifically activate IT or PT neurons. At the same time, we used patch-clamp recordings to monitor the effect of IT or PT activation in the postsynaptic neurons in the striatum. We combined this with pharmacology to understand the specific mechanisms involved in the signals we evoked.
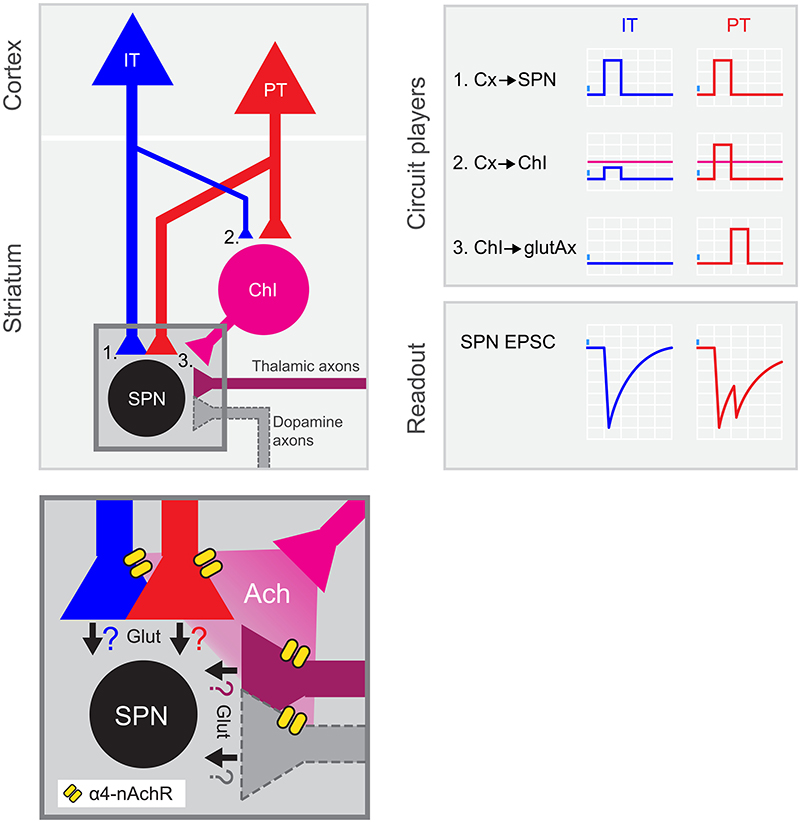
Figure 6. PT neurons amplify excitatory inputs to striatum through ChIs. Summary of the results: left, circuit diagram proposed for corticostriatal connectivity. IT and PT cortical neurons project to both SPNs (1) and ChIs (2). While PT➔SPN (1, red) and PT➔ChI (2, red) input strength is similar, IT➔ChI (2, blue) connection is weaker than IT➔SPN (1, blue). Within the striatum, ChIs convey excitation to SPNs by recruiting long-range glutamate-releasing terminals reaching DLS through α4-containing nicotinic receptors (3 and inset). Inset shows the putative axonal sources of glutamate release during the second EPSC peak. Ach, acetylcholine; α4-nAchR, α4-containing nicotinic receptors; Glut, glutamate. Right: Schematic of the activation of the different circuit players upon IT or PT photostimulation and its impact on the recorded SPNs. Magenta horizontal lines represent ChI spiking threshold.
As recently reported in Science Advances, we found a new circuit motif involving three different cell types linked to one of the corticostriatal pathways we studied, the pyramidal tract. This previously unknown motif runs in parallel to the canonical connection from cortex to spiny projection neurons (SPNs). This motif is strongly biased towards PT and not IT neurons, and contact striatal cholinergic interneurons that, in turn, indirectly excite SPNs by promoting glutamate release from long-range axons reaching the striatum. We showed that alfa4-contanining nicotinic receptors, a subtype of acetylcholine receptors, are responsible for this mechanism.
This research advances a small but necessary step towards understanding the wiring of the brain circuits controlling animal behavior. Tackling these circuits could have implications for understanding learning as well as disorders that involve the basal ganglia, such as Parkinson's disease.
Inbal Israely, PhD
Assistant Professor of Pathology and Cell Biology (in Neuroscience and the Taub Institute)
ii2176@cumc.columbia.edu

| 
|
Davangere P. Devanand, MBBS, MD | Seonjoo Lee, PhD |

| 
|
Edward D. Huey, MD | Terry E. Goldberg, PhD |
Neuropsychiatric symptoms (NPS) frequently occur in Alzheimer's Disease and Related Dementias (ADRD), are difficult to treat, and often indicate a poor prognosis. The associations of NPS with clinical diagnostic subtypes have been studied extensively. Less is known about these associations with neuropathological diagnoses. Together with my colleagues Drs. Seonjee Lee, Edward Huey, and Terry Goldberg, we evaluated the associations of brain autopsy ADRD diagnoses with Neuropsychiatric Interview-Questionnaire (NPI-Q) symptom domains in a study of 1,808 brains from 39 sites in the National Alzheimer’s Coordinating Center (NACC).
As recently reported in JAMA Psychiatry, we found that delusions and hallucinations were common in Alzheimer's Disease (AD) and Lewy Body Disease (LBD) and less common in Frontotemporal Lobar Degeneration (FTLD), which was associated with greater disinhibition and apathy. The highest prevalence for any NPI-Q symptom domain was apathy, which reached 80% in hippocampal sclerosis. The associations between hippocampal sclerosis and increased apathy and disinhibition remained after excluding individuals with FTLD. Patients with both LBD and AD showed a higher prevalence of delusions, hallucinations and agitation/aggression compared to LBD without AD. Across diagnoses, more severe pathology was associated consistently with increased NPS, indicating that NPS reflect underlying ADRD pathology (e.g., LBD showed increase in hallucinations from brainstem to limbic to neocortical pathology). Symptoms of apathy and disinhibition in the diagnostic criteria for behavioral variant FTLD were supported. In hippocampal sclerosis, which has rarely been studied for neuropsychiatric symptoms, our novel findings of increased apathy and disinhibition merit further investigation. These findings can inform clinicians who diagnose and treat NPS.
Davangere P. Devanand, MBBS, MD
Professor of Psychiatry (in Neurology and the Gertrude H. Sergievsky Center) at CUMC
dpd3@cumc.columbia.edu
 |  |  |
||
| Laura Zahodne, PhD | Jennifer J. Manly, PhD | Adam M. Brickman, PhD |
Non-Hispanic Black older adults experience disproportionate burden of Alzheimer’s disease and related dementias (ADRD) compared with non-Hispanic Whites, and these inequalities are only partially explained by socioeconomic factors like education and income. An emerging literature points to other modifiable experiential factors, such as racial discrimination.
Recently, together with Taub Institute colleagues Drs. Adam Brickman, Jennifer J. Manly, and others, we added a battery of psychosocial measures to the long-running Washington Heights-Inwood Columbia Aging Project to more fully identify factors that create and sustain ADRD inequalities. In a study to be published in Social Science & Medicine, we investigated which types of discrimination are most likely to affect brain aging among 221 non-Hispanic Black older adults who underwent magnetic resonance imaging (MRI). We found that aggregate measures of discrimination that collapse across attributions (e.g., race, age, gender, height/weight, sexual orientation, physical disability) were not associated with brain aging, but discrimination specifically attributed to race and/or skin color was associated with both hippocampal volume and white matter hyperintensities (WMH). This pattern of results suggests that racial discrimination may be particularly detrimental to brain health among non-Hispanic Black older adults. Future work on discrimination and health disparities should disaggregate reports of discrimination by attribution, as disregarding the identified reasons for discrimination could result in underestimation of its negative health effects.
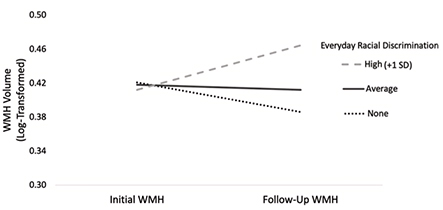
Figure 2.Changes in WMH as a function of everyday racial discrimination, adjusted for covariates. Note. WMH = White matter hyperintensities; SD = Standard deviation.
We also found that different types of discrimination were associated with different MRI outcomes. Specifically, racial discrimination in major life domains (e.g., labor market, lending, housing, criminal justice) predicted lower initial hippocampal volume, while everyday racial discrimination (e.g., microaggressions) predicted faster accumulation of WMH over four years. This pattern of findings may point to different mechanisms underlying the negative health effects of structural versus interpersonal racism. Structural racism shapes an individual’s life course opportunities and environments, which could have a negative impact on hippocampal development and maintenance leading up to late life. As just one example, racial residential segregation determines lifetime exposure to air pollution and other environmental toxicants known to be particularly deleterious to hippocampal development. In contrast, the continued experience of interpersonal racism during late life represents a chronic stressor that could have a negative impact on inflammatory pathways linked to worse cardiovascular and cerebrovascular health.
Given that both hippocampal volume and WMH predict incident ADRD, these results highlight how racism may contribute to racial inequalities in ADRD in a variety of ways. Studies of brain and cognitive aging should incorporate measures of racial discrimination to understand biopsychosocial pathways linking racism to ADRD risk and to identify points of intervention.
Laura B. Zahodne, PhD
Assistant Professor of Psychology, University of Michigan
lzahodne@umich.edu
The Penalty of Stress - Epichaperomes Negatively Reshaping the Brain in Neurodegenerative Disorders

Ottavio Arancio, MD, PhD
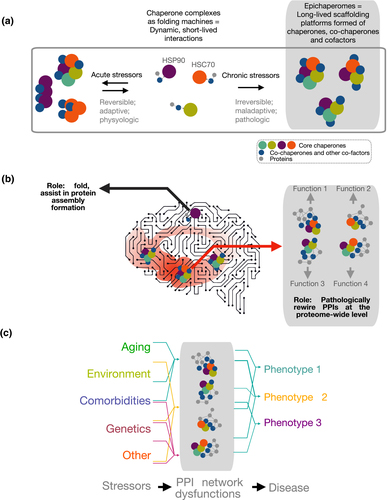
Figure 1. Biochemical (a) and functional (b, c) distinction between chaperones and epichaperomes. See full caption.
Adaptation to acute and chronic stress and/or persistent stressors is a subject of wide interest in central nervous system disorders, including Alzheimer’s Disease (AD). This review, recently published in the Journal of Neurochemistry, is the result of a combined effort by my Taub Institute laboratory and that of Dr. Chiosis at Memorial Sloan Kettering. Our two laboratories are investigating how the organism and the brain respond to a potential threat. The hypothesis is that upon entry of oligomeric tau and Aβ species—the two major toxic molecules in AD—the molecular stress triggered induces a maladaptive switch of the chaperome into epichaperomes.
Chaperomes, which form short-lived dynamic complexes with individual proteins and are found in all cells, safeguard how individual proteins get made and folded. Conversely, epichaperomes are stabilized heterooligomeric complexes specific to cells exposed to chronic stressors; they do not fold but rather change how proteins interact with each other. They act as pathologic scaffolds to cause thousands of proteins to improperly organize inside cells, aberrantly affecting cellular phenotypes. It is expected that these studies will deliver proteome-wide functional insights and comprehensive, mechanistic understanding into how Aβ and tau oligomers lead to synaptic failure and cognitive defects. In addition to providing new insights into AD biology, these studies have also an immediate translational application, as there is a therapeutic that inhibits epichaperomes now in Phase 1 clinical evaluation.
Ottavio Arancio, MD, PhD
Professor of Pathology and Cell Biology (in the Taub Institute)
oa1@cumc.columbia.edu

