Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
March 2022
2: Correlation of Plasma and Neuroimaging Biomarkers in Alzheimer's Disease
3: Probing the Proteome to Explore Potential Correlates of Increased Alzheimer's-Related Cerebrovascular Disease in Adults with Down Syndrome
Homotypic Fibrillization of TMEM106B Across Diverse Neurodegenerative Diseases

Anthony W.P. Fitzpatrick, PhD

Pictured are four of the six co-first authors:(from left to right): Marija Simjanoska, Andrew Chang, Carolyn Lee, and Xinyu Xiang.
Misfolding and aggregation of disease-specific proteins, resulting in the formation of filamentous cellular inclusions, is a hallmark of neurodegenerative disease with characteristic filament structures, or conformers, defining each proteinopathy. Here we show that a previously unsolved amyloid fibril comprised of a 135 amino acid C-terminal fragment of TMEM106B is a common finding in distinct human neurodegenerative diseases, including cases characterized by abnormal aggregation of TDP-43, tau, or -synuclein protein. A combination of cryo-electron microscopy and mass spectrometry was used to solve the structures of TMEM106B fibrils at a resolution of 2.7 Å from postmortem human brain tissue afflicted with frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP, n=8), progressive supranuclear palsy (PSP, n=2), or dementia with Lewy bodies (DLB, n=1). The commonality of abundant amyloid fibrils composed of TMEM106B, a lysosomal/endosomal protein, to a broad range of debilitating human disorders indicates a shared fibrillization pathway that may initiate or accelerate neurodegeneration.
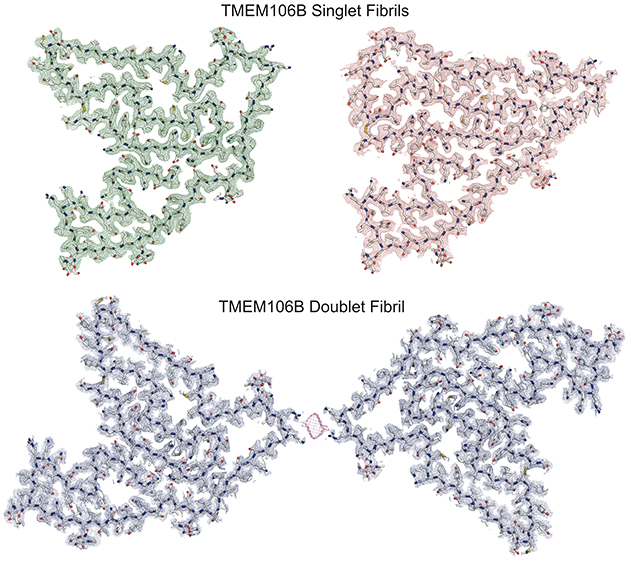
Figure 1. A key fragment of the protein TMEM106B, several atomic models of which are shown here, can stack into single or twinned types of fibrils. Credit: Andrew Chang and Anthony Fitzpatrick / Columbia University's Zuckerman Institute / Cell.
As recently reported in Cell, the fibrillization of TMEM106B into identical fibrillar structures in a wide range of sporadic or genetic TDP-43 proteinopathies (FTLD-TDP), a tauopathy (PSP), and a synucleinopathy (DLB) points toward a commonality between these diverse neurodegenerative diseases that raises many intriguing questions.
While future experiments will determine whether TMEM106B (120–254) fibril formation represents a loss or gain of function, our findings identify a common fibrillization pathway and widespread structural target that may be implicated in neurodegeneration.
Anthony W.P. Fitzpatrick, PhD
Assistant Professor of Biochemistry and Molecular Biophysics (in the Zuckerman Institute and the Taub
Institute)
Anthony.Fitzpatrick@columbia.edu
Correlation of Plasma and Neuroimaging Biomarkers in Alzheimer's Disease
 |  |  | ||
| Adam M. Brickman, PhD | Jennifer J. Manly, PhD | Richard Mayeux, MD, MSc |
In collaboration with Drs. Jennifer J. Manly, Richard Mayeux, and colleagues from Taub, we previously showed that blood concentrations of phosphorylated tau at threonine 181 and 217 (Ptau 181 and Ptau 217) along with neurofilament light (NfL) chain were elevated in a cohort of multi-racial/ethnic, older adults diagnosed clinically and pathologically with Alzheimer’s disease (AD), from the community-based, Washington Heights-Inwood Columbia Aging Project (WHICAP). We also found that increased Ptau 181 and Ptau 217 concentrations and decreased Aβ42/Aβ40 ratios were associated with higher risk of AD after an average of 4 years. This research adds to a growing body of evidence that suggests blood-based AD biomarker concentrations are associated with pathological and clinical diagnoses and can predict future development of clinical AD, providing evidence that they can be incorporated into multiethnic, community-based studies.
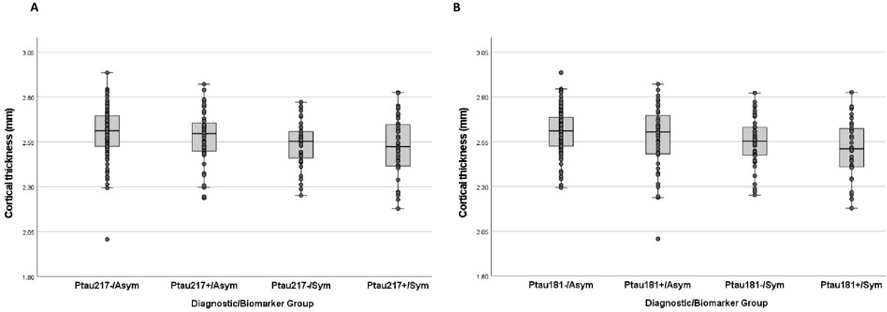
The focus of this follow-up study was to examine demographic (i.e., age, sex/gender, race/ethnicity), genetic (i.e., APOE ε4 allele), amyloid (Aβ42/Aβ40), tau (total tau concentration), and neurodegeneration (NfL, cortical atrophy) variables across older adults classified as biomarker positive (Ptau+) or negative (Ptau-) based on Ptau 181 and 217 concentrations, and as cognitively impaired (Sym) or unimpaired (Asym). Three hundred participants from the WHICAP cohort were selected for equal representation from race/ethnicity groups: non-Latinx White, Latinx/Hispanic, and non-Latinx African American/Black), with similar numbers with an AD clinical diagnosis at their last longitudinal assessment. A goal for the current analyses was to define four groups based on symptom and biomarker status.
|
Figure 1. Differences across biomarker/clinical groups in cortical thickness in Alzheimer's signature regions for groups defined by Ptau 217 cutpoint (A) and by Ptau 181 cutpoint (B).
|
As recently reported in the Annals of Clinical and Translational Neurology, the four groups identified from this racial/ethnically diverse cohort study—Ptau-/Asym, Ptau+/Asym, Ptau-/Sym, and Ptau+/Sym—differed by age, APOE-4 allele frequency, total tau, neurofilament light chain, and cortical thickness measured by MRI. These results add to increasing evidence that plasma Ptau 181 and 217 concentrations are valid Alzheimer's disease biomarkers in diverse populations.
Adam M. Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  | |
| Fahmida Moni | Adam M. Brickman, PhD |
Cerebrovascular disease is associated with symptoms and pathogenesis of Alzheimer's disease (AD) among adults with Down syndrome (DS). Because individuals with DS do not exhibit the classical vascular risk factors that promote cerebrovascular disease, the cause of increased dementia-related cerebrovascular disease in DS is unknown. Previous research from the our laboratory found that cerebrovascular lesions—including white matter hyperintensities (WMH), enlarged perivascular spaces (PVS), infarcts, and microbleeds—were detectable among adults with DS as early as age 40 and increased in severity with the diagnosis of mild cognitive impairment and clinical AD.
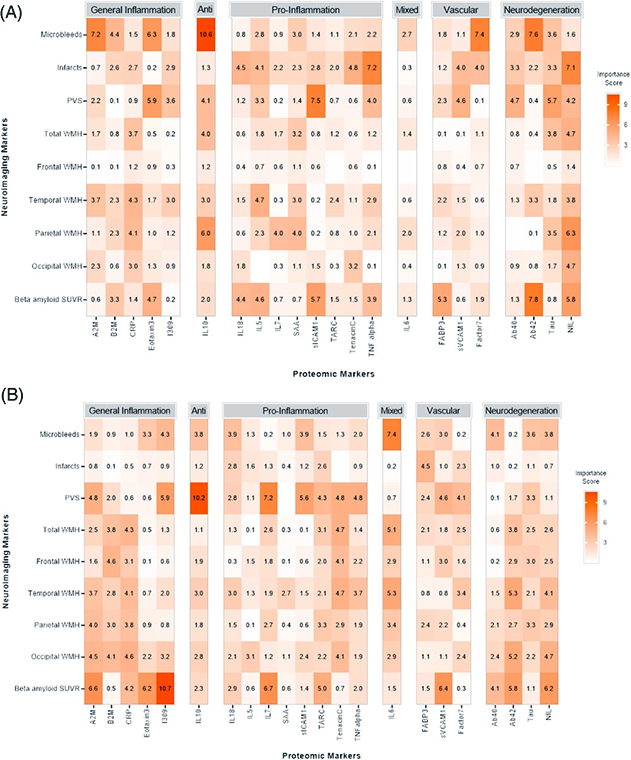
Figure 1. Summary of variable important plots. Each row in the heat map represents a separate analysis with numeric variable importance scores displayed and color coded (more saturated reds represent greater relative importance). Top figure (A) includes participants with cognitive impairment and the bottom figure (B) includes those with stable cognitive functioning. Protein markers are grouped by general function, including general inflammation, anti-inflammatory, pro-inflammatory, mixed anti-/pro-inflammatory, vascular, and Alzheimer's disease/neurodegeneration. Anti, anti-inflammation; Mixed, mixed anti-/pro-inflammation.
Building on this research, together with co-first authors Fahmida Moni and Melissa Petersen, we explored whether protein markers of neuroinflammation are associated with markers of cerebrovascular disease among adults with DS. Participants from the Alzheimer's disease in Down syndrome (ADDS) study, led by Taub colleague Dr. Nicole Schupf, with magnetic resonance imaging (MRI) scans and blood biomarker data were included. Support vector machine (SVM) analyses examined the relationship of blood-based proteomic biomarkers with MRI-defined cerebrovascular disease among participants characterized as having cognitive decline and as being cognitively stable.
As recently reported in Alzheimer’s & Dementia, we found that inflammatory and AD markers were associated with cerebrovascular disease, particularly among symptomatic individuals with DS. The pattern of results suggested a strong inflammatory component to the observed cerebrovascular lesions with relatively greater AD pathophysiology involvement among those with cognitive decline. These findings help to generate hypotheses that both inflammatory and AD markers are implicated in cerebrovascular disease among those with DS, and point to potential mechanistic pathways for further examination.
Notably, this project was initiated by Fahmida Moni while a scholar in the STAR U Summer of Translational Aging Research Program, led by Drs. Brickman and Cosentino.
Adam M. Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu