Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2022
2: Wolframin is a Novel Regulator of Tau Pathology and Neurodegeneration
3: Clinical Trajectories at the End of Life in Dementia Patients With Alzheimer Disease and Lewy Body Neuropathologic Changes
Progranulin Mutations in Clinical and Neuropathological Alzheimer's Disease
 |  | |
| Badri N. Vardarajan, PhD, MS | Richard Mayeux, MD, MSc |
Progranulin (GRN) is a multifunctional protein, predominantly produced and secreted by the microglia. GRN mutations occur in frontotemporal lobar degeneration (FTLD) and explain up to 20 percent of familial and 5 percent of sporadic FTLD. They lead to a variety of clinical presentations, predominantly presenting as behavioral variant FTLD or progressive aphasia. Recent genetic and epidemiological studies suggest that GRN variants may also be observed in AD often with TDP-43 pathology. Decrease in GRN expression increases AĪ² and tau deposition in mice and is over-expressed in the microglia surrounding plaques.
In this recently published Alzheimerās & Dementia study, we investigated the frequency of pathogenic GRN mutations in unrelated and familial AD cohorts with either clinical or postmortem AD. In addition, we also studied GRN variant rs5848, which is associated with a 3.2-fold increased risk for ubiquitin- and TAR DNA-binding protein 43 (TDP-43)-positive frontotemporal dementia (FTLD-U). In clinical AD, we compared the frequency of behavioral and other symptoms (such as learning disabilities) consistent with a FTLD presentation. In autopsied-confirmed AD, we evaluated the presence of co-pathologies including tauopathies and TDP-43 presentation.
|
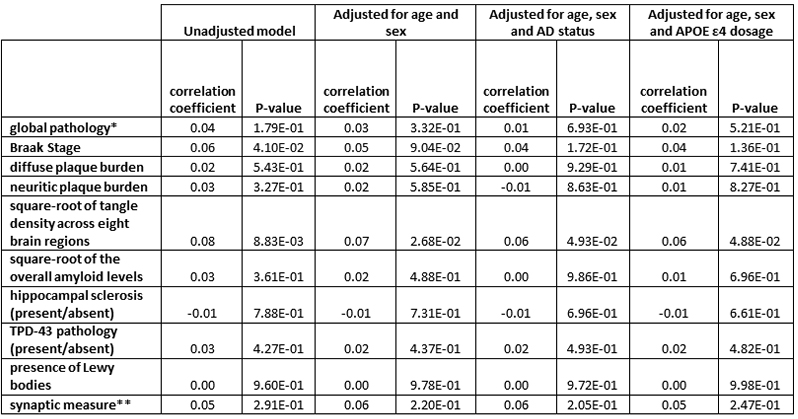
Table 1: GRN carrier status is correlated positively with tangle load and Braak stage
* global pathology defined as global measure of pathology based on the scaled scores of pathology in 5 brain regions, where the scaled variable is the original count divided by the standard deviation ** synaptic measure across three cortical (hippocampus, midfrontal cortex, and inferior temporal |
We found higher than expected frequency of pathogenic GRN mutations among autopsied and clinically diagnosed AD compared to publicly available exome and genome datasets (GnomAD). However, we did not find any evidence for association with AD. Pathogenic GRN carriers had significantly higher PHFtau tangle density adjusting for age, sex and APOEĪµ4 genotype. AD patients with the rs5848 mutation had higher frequency of hippocampal sclerosis and TDP-43 deposits. In Hispanic families, we found learning disabilities and aphasia concomitant with clinical AD in GRN carriers, but this was absent in non-carriers. GRN variants are present in ~16% primary progressive aphasia (PPA), 7% of behavioral-FTLD and ~5% of AD with learning disabilities suggesting that increased language and behavioral deficits in the presence of GRN variants in clinically diagnosed AD.
Badri N. Vardarajan, PhD, MS
Assistant Professor of Neurological Science (in Neurology, the Gertrude H. Sergievsky Center, and the Taub Institute)
bnv2103@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Taub Institute and the Gertrude H. Sergievsky Center)
rpm2@cumc.columbia.edu
Wolframin is a Novel Regulator of Tau Pathology and Neurodegeneration
 |  | |
| Karen Duff, PhD | Hongjun (Harry) Fu, PhD |
In collaboration with Drs. Karen Duff, Abid Hussaini, and colleagues from the Taub Institute, we previously showed that grid cells (a cluster of excitatory neurons in layers II/III of the entorhinal cortex that form part of the spatial navigation system) are specifically vulnerable to pathologic tau accumulation, resulting in grid cell dysfunction and associated spatial memory deficits in a tau transgenic mouse model. Interestingly, one of the molecular characteristics of grid cells in the entorhinal cortex is a strong expression of wolframin (WFS1). The focus of this follow-up study was to examine if and why WFS1-expressing excitatory neurons are particularly vulnerable to tau pathology and neurodegeneration.
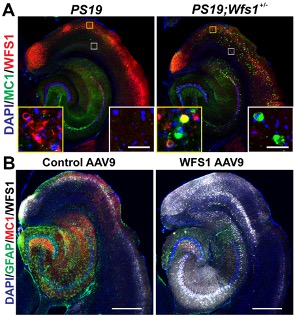
Figure 1. WFS1 deficiency is associated with increased tau pathology in PS19 tau mice (A), whereas overexpression of WFS1 reduces the tau pathology and astrogliosis in PS19 tau mice (B). [Courtesy of Chen et al., Acta Neuropathologica, 2022.]
As recently reported in Acta Neuropathologica, we found WFS1-expressing excitatory neurons in layers II/III of the entorhinal cortex are vulnerable in tau mouse models, human Alzheimerās disease (AD) and other types of proteinopathies, including frontotemporal lobar degeneration with tau pathology (FTLD-Tau), frontotemporal lobar degeneration with transactive response DNA-binding protein (FTLD-TDP), and diffuse lewy body disease (DLBD). WFS1 deficiency is associated with increased tau pathology, astrogliosis, postsynaptic degeneration, apoptosis and cognitive deficits in PS19 tau mice, whereas overexpression of WFS1 reduces those changes. We also find that WFS1 interacts with tau protein and controls the susceptibility to tau pathology. Furthermore, chronic ER stress- and autophagy-lysosome pathway (ALP)-associated genes are enriched in WFS1-high excitatory neurons in human AD at early Braak stages. The protein levels of ER stress- and autophagy-lysosome pathway (ALP)-associated proteins are changed in tau transgenic mice with WFS1 deficiency, while overexpression of WFS1 reverses those changes.
This work demonstrates a novel role for WFS1 in the regulation of tau pathology and neurodegeneration via chronic ER stress and the downstream ALP. Our findings provide insights into mechanisms that underpin selective neuronal vulnerability, and for developing new therapeutics to protect vulnerable neurons in AD and other types of proteinopathies. Results from this study were also featured on OSUMC Press release this month.
Hongjun (Harry) Fu, PhD
Assistant Professor
Department of Neuroscience, The Ohio State University
Hongjun.Fu@osumc.edu
Karen Duff, PhD
Centre Director, UK Dementia Research Institute at UCL
Professor Emerita, Columbia University Irving Medical Center
k.duff@ucl.ac.uk
Lawrence S. Honig, MD, PhD
Professor of Neurology (in the Taub Institute and the Gertrude H. Sergievsky Center)
lh456@cumc.columbia.edu
Jean Paul Vonsattel
Professor of Pathology and Cell Biology (in the Taub Institute and the New York Brain Bank)
jgv2001@cumc.columbia.edu
 |  | |
| Yian Gu, MD, MS, PhD | Yaakov Stern, PhD |
Inconsistent findings have been reported with regard to the disease courses of the two most common dementias, Alzheimerās disease (AD) and Dementia with Lewy bodies (DLB). Part of this inconsistency may be due to changes over time in clinical diagnosis guidelines. Furthermore, past studies have relied mainly on clinical diagnoses which likely represent a mixture of underlying pathologies, and longitudinal studies are scarce. It remains unclear whether the disease trajectory in the years before death differs among dementia patients with pure Alzheimerās disease (AD), pure Dementia with Lewy bodies (DLB), or mixed (AD and DLB) pathologies.
In the current study, we aimed to clarify this question using autopsy-confirmed diagnoses of pure AD (n=34), mixed AD and DLB (AD+DLB, n=17), or pure DLB (n=11) from the Predictors 2 Cohort Study, a prospective, clinic-based, cohort of dementia patients. Our study used Folstein Mini-Mental State Examination (MMSE) as a measure of cognition, and Activities of Daily Living (ADL) and dependence scale (DS) to measure functional abilities. The cognitive and functional trajectories over time were compared across the three patient groups, controlling for age, sex, education, and baseline features including extrapyramidal signs, MMSE, ADL, and DS.
|
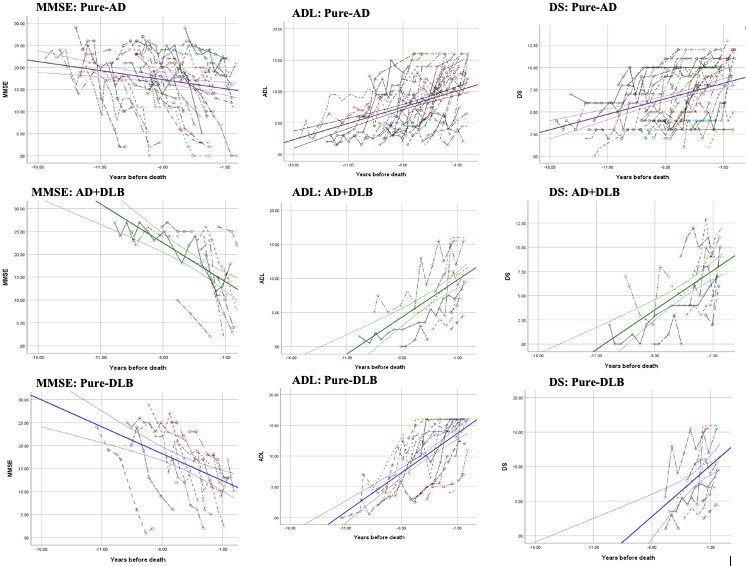
Figure 1. Cognition and functional change in the years preceding death in autopsy-confirmed dementia patients.
The figures show cognition (MMSE) and functional (ADL, DS) changes in the years preceding death in autopsy-confirmed dementia patients, for dementia patients with pure-AD (purple), AD+DLB (green), or pure-DLB (blue). Each colored dashed line shows the observed MMSE (first column), ADL (second column), and DS (third column) scores for each individual. Abbreviations: MMSE, miniāmental state examination; ADL, activities of daily living; DS, dependence scale. |
As recently reported in Neurology, we found that all patients experienced a decline in both cognition and function over a mean 5.4Ā±2.9 years of follow-up at 6-month intervals. Furthermore, compared to the pure AD group, the pure DLB group experienced approximately double the rate in both MMSE and functional DS decline, and the AD+DLB group showed double the rate in decline of ADL. Overall, the study found dementia patients with Lewy body pathology experienced faster cognitive and functional decline than pure-AD patients. The findings of this autopsy-based study have great implications for clinical management, future clinical study design, and research on pathology-specific biomarkers.
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology in the Gertrude H. Sergievsky Center and the Taub Institute)
yg2121@cumc.columbia.edu