Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
August 2015
» #2 Brain Amyloid Deposition and Longitudinal Cognitive Decline in Nondemented Older Subjects: Results from a Multi-Ethnic Population.
Stereotaxic Infusion of Oligomeric Amyloid-beta into the Mouse Hippocampus.
 |  |
| Carol M. Troy, MD, PhD | Ying Y. Jean, PhD |
A critical factor in the study of human disease is the availability of appropriate models with which to study the mechanisms of the disease. Optimally, models would replicate the human pathology. Such models have been difficult to develop for the study of Alzheimer's Disease (AD). Multiple transgenic models have been developed which only selectively mimic the pathology of AD. Most notably, neuronal death, a prominent hallmark in the brains of Alzheimer's patients, is lacking in the various genetic models. Our laboratory focuses on the study of the molecular mechanisms of neuronal death and we aimed to develop a model that would replicate the loss of neurons found in AD.
In our recent publication in JoVE (Journal of Visualized Experiments), we provide an alternative mouse model in which we induce neuronal loss using stereotaxic guided convection enhanced delivery of oligomeric amyloidβ1-42 to targeted brain regions. Convection enhanced delivery utilizes pressure to deliver larger volumes more uniformly to the brain parenchyma. This model provides spatial and temporal regulation of the levels of oligomeric Aβ1-42, the species of Aβ which is most toxic to neurons. This study describes in detail the method used in our previous studies.
In the accompanying video, the infusion target is the dentate gyrus of the hippocampus because this area, along with the basal forebrain which is connected by the cholinergic circuit, represents one of the areas of degeneration in the disease. The results of elevating Aβ1-42 in the dentate gyrus via stereotaxic infusion reveal increases in neuron loss in the dentate gyrus within 1 week, while there is a concomitant increase in cell death and cholinergic neuron loss in the vertical limb of the diagonal band of Broca of the basal forebrain, shown in the accompanying figures. These effects are observed up to 2 weeks. Hence, this in vivo model serves as an excellent model to investigate the semi-acute effects of Aβ1-42 locally.
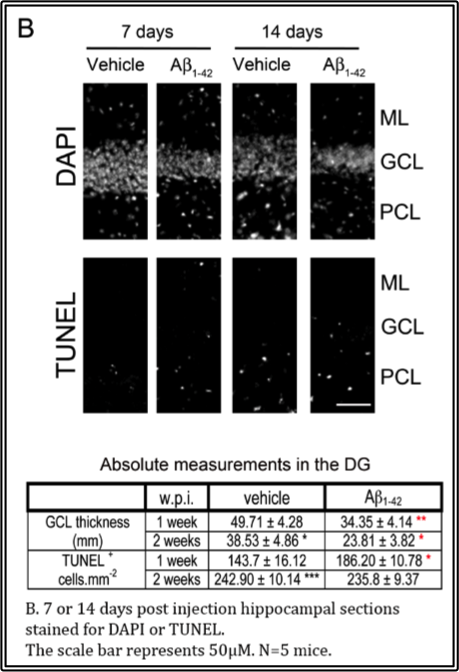 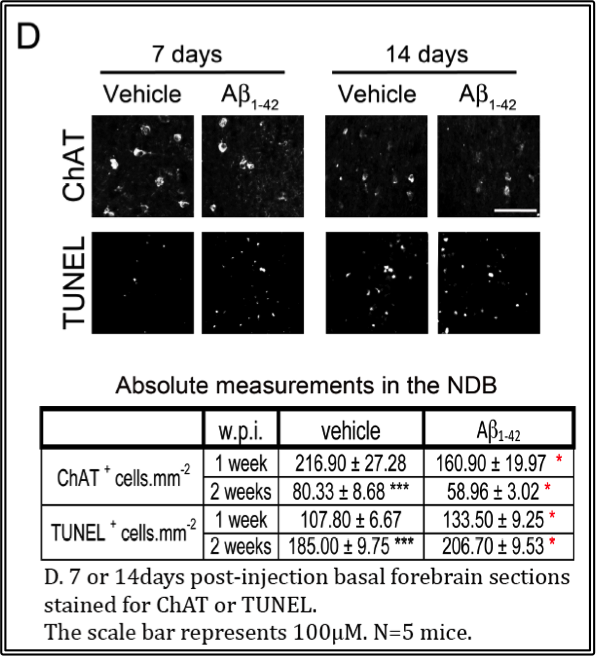 |
The key advantage of this injection model is its dynamic nature: any brain region may be targeted and different aged animals may be used. 9 month-old male mice were chosen in our experiments because we believe increasing Aβ1-42 levels in older mice better simulates the disease which occurs in older people. Moreover, we aim to keep the experiments consistent by using mice of the same age. However, mice of any age could be used for the injection. In the past, we have experimented on mice ranging from 2 to 16 months old. This method can also be used for introducing other compounds of interest, including viruses, siRNA and other peptides/proteins, into wild-type animals.
Carol M. Troy, MD, PhD
Professor of Pathology, Cell Biology and Neurology (in the Taub Institute)
cmt2@cumc.columbia.edu
Ying Y. Jean, PhD
Department of Pathology and Cell Biology
yyj2103@cumc.columbia.edu
 |  |
| Yian Gu, MD, MS, PhD | Yaakov Stern, PhD |
A hallmark of Alzheimer's disease (AD), the leading cause of dementia in the elderly, is the deposit of amyloid-β (Aβ) in the brain. However, a 20%~30% prevalence of Aβ deposition in brain has been seen among cognitively normal, asymptomatic elderly using in vivo positron emission tomography (PET) imaging of radioligands that bind to fibrillar Aβ in amyloid plaques. It remains unclear whether the abnormally high Aβ level in the brain among apparently healthy elderly people indicates an underlying subtle cognitive deficit and/or accelerated cognitive decline.
In a study recently published in PLOS ONE, Drs. Yian Gu, Yaakov Stern, and colleagues from the Taub Institute examined the relationship between PET-measured cerebral Aβ level and longitudinal cognition change up to 20 years before the PET. In this study, a total of 116 dementia-free participants (mean age 84.5 years) of the Washington Heights-Inwood Community Aging Project (WHICAP) completed 18F-Florbetaben PET imaging. Positive or negative cerebral Aβ deposition was assessed visually. Quantitative cerebral Aβ burden was calculated as the standardized uptake value ratio in pre-established regions of interest using cerebellar cortex as the reference region. Cognition was determined using a neuropsychological battery, and selected tests scores were combined into four composite scores (memory, language, executive/speed, and visuospatial) using exploratory factor analysis. The study found 41 of 116 (35%) individuals had positive reading of Aβ. Aβ positive reading (B = -0.034, p = 0.02) and higher Aβ burden in temporal region (B = -0.080, p = 0.02) were associated with faster decline in executive/speed. Stratified analyses showed that higher Aβ deposition was associated with faster longitudinal declines in mean cognition, language, and executive/speed in African-Americans or in APOE ε4 carriers, and with faster memory decline in APOE ε4 carriers. The associations remained significant after excluding mild cognitive impairment participants.
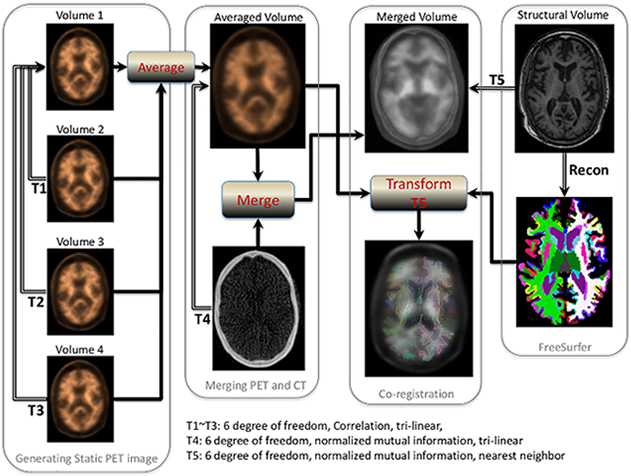 |
Overall, this study found that higher Aβ deposition in healthy elders was associated with faster decline in executive/speed in the decade before neuroimaging, and the association was observed primarily in African-Americans and APOE ε4 carriers. These findings suggest that measuring cerebral Aβ may give us important insights into the cognitive profile in the years prior to the scan in cognitively normal elders.
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology and the Taub Institute)
yg2121@columbia.edu
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center, and in the Taub Institute)
ys11@columbia.edu

