Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
July 2025
2: Phase Contrast-Derived Cerebral Blood Flow is Associated with Neurodegeneration and Cerebrovascular Injury in Older Adults
3: Accelerating Biomedical Discoveries in Brain Health Through Transformative Neuropathology of Aging and Neurodegeneration
 |  | |
| Richard Mayeux, MD, MSc | Mark Logue, PhD (Boston University) |
As part of a multi-institutional study led by researchers from Boston University, we recently explored how genetic risk for Alzheimer’s disease may differ across subgroups within African American populations. Using genome-wide association data from both the VA Million Veteran Program and the Alzheimer’s Disease Genetics Consortium, we stratified analyses by sex, age at onset, and APOE-ε4 status—factors that are often biologically meaningful but underexamined in combination, particularly in non-European cohorts.
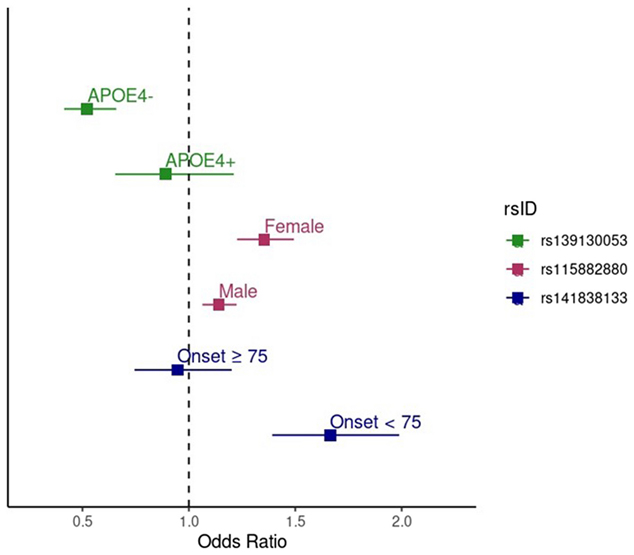
Figure 2. Forest plot showing the effect sizes (odds ratios) and 95% confidence intervals for the MVP/ADGC meta-analysis results for the genome-wide significant SNPs in both the stratum in which the association was identified and the opposite stratum. The green points show the effect of rs139130053 in APOE-ε4 negative individuals (upper green point) and APOE-ε4 positive individuals (lower green point). The pink points show the effect of rs115882880 in females (upper pink point) and males (lower pink point). The blue points show the effect of rs141838133 in individuals with onset age ≥ 75 years (upper blue point) and individuals with onset age < 75 years (lower blue point).
As recently reported in Alzheimer’s Research & Therapy, this approach uncovered three genome-wide significant loci outside of the APOE region: a variant near EPHA5 associated with earlier onset cases, a signal in GRIN3B (next to ABCA7) that was strongest in females, and a variant near TSPEAR in APOE-ε4 non-carriers. These genes are implicated in pathways related to synaptic plasticity, insulin signaling, and protein degradation—mechanisms that are increasingly recognized as relevant to Alzheimer’s disease pathophysiology.
These findings add to growing evidence that stratified genetic analysis—particularly in underrepresented populations—can reveal novel risk loci that may be missed in more generalized approaches. They underscore the importance of both diverse population representation and biologically informed subgroup analysis in dementia genetics. Together, these strategies offer a more nuanced view of Alzheimer’s disease risk and help illuminate the complexity of its genetic basis across different groups.
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Sergievsky Center and Taub Institute)
rpm2@cumc.columbia.edu
 |  | |
| Jeffrey Pyne, PhD | Adam Brickman, PhD |
Using data from the Washington Heights-Inwood Columbia Aging Project (WHICAP), our recent study, led by Dr. Jeffrey Pyne, examined how extracranial blood flow relates to structural markers of brain health in older adults. Phase contrast MRI was used to measure blood flow through the major arteries supplying the brain, and we assessed its associations with cortical volume, white matter integrity, white matter hyperintensities (WMH), and cerebral microbleeds in a racially and ethnically diverse cohort of 311 participants.
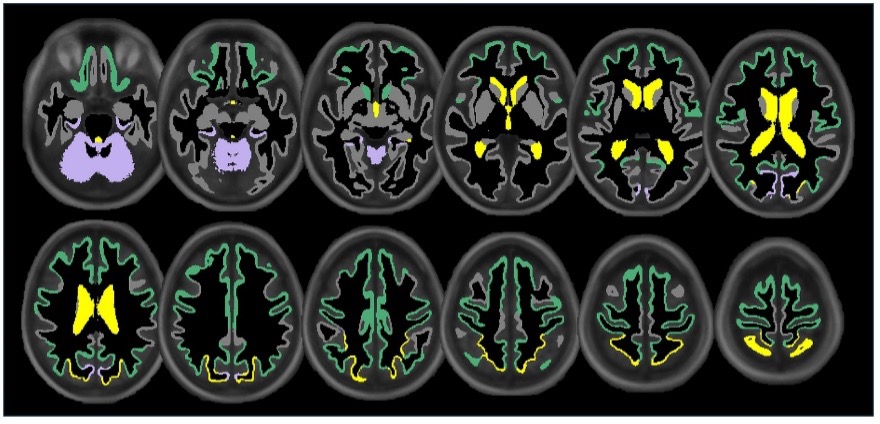
Figure 3. Illustration showing the significant associations of extracranial total (yellow), anterior (green), and posterior (purple) cerebral blood flow with cortical, subcortical, and ventricular system regional volume ROIs. Regional volume ROIs with nonsignificant associations with extracranial blood flow are shown in gray. Analyses are adjusted for age, vascular risk factors, intracranial volume, and sex, with corrections applied for multiple comparisons.
As recently reported in Frontiers in Neuroscience, lower extracranial cerebral blood flow—both total and within the anterior and posterior circulations—was associated with reduced white matter tract integrity in key regions such as the forceps minor, cingulum cingulate gyrus, and inferior fronto-occipital fasciculus. We also observed that reduced blood flow was linked to smaller cortical volumes and greater WMH burden, suggesting that diminished vascular supply may contribute to both neurodegeneration and small vessel disease.
These findings support the view that systemic vascular factors—such as reduced extracranial blood flow—may play a meaningful role in driving structural brain changes associated with aging and cerebrovascular disease. Longitudinal research is needed to determine the temporal dynamics and causal direction of these associations.
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute, and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu

In this recently published Neuron perspective, co-led by Dr. Melissa Murray (Mayo Clinic Jacksonville), we explore how the field of neuropathology is being reshaped by a convergence of technologies and disciplines. What we call “transformative neuropathology” goes beyond integrating traditional histopathology with advanced tools like multi-omics and machine learning—it’s about building a framework that allows us to extract much deeper insights from postmortem human brain tissue. These insights are critical for identifying disease mechanisms, biomarkers, and therapeutic targets. However, advancing this kind of research also depends on strengthening the systems around it: sustaining brain banks, broadening donor participation, accelerating autopsy workflows, and improving how we train the next generation of investigators.
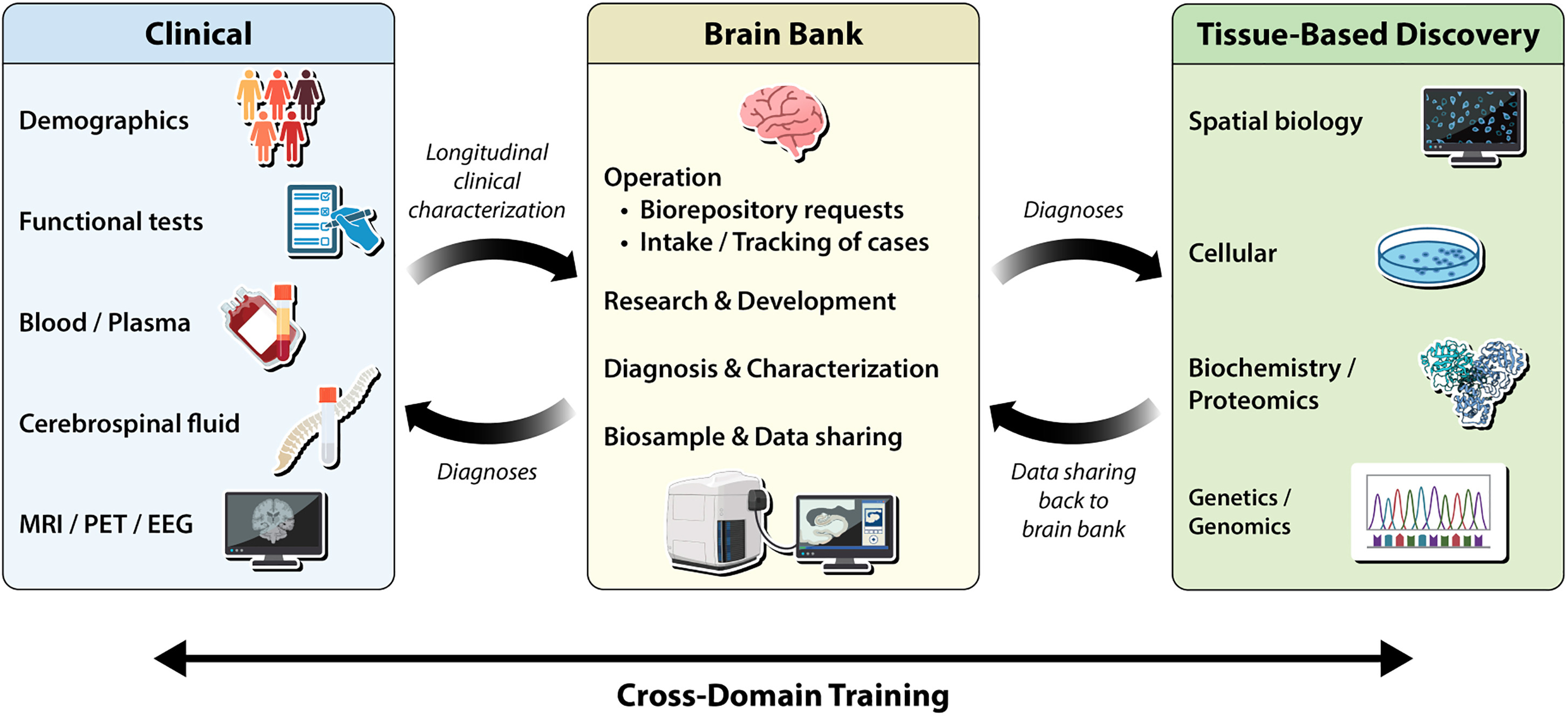
Figure 2. Overview of a brain bank ecosystem. Three boxes depict the ideal brain bank ecosystem with bidirectional flow of information and cross-domain training toward accelerating biomedical discoveries. See full caption.
Working closely with Taub Institute investigators Drs. Vilas Menon, Philip L. De Jager, and a team of collaborators from multiple institutions, we focused on practical, forward-looking ways to enhance how we collect, share, and analyze human brain tissue. This includes everything from improving tissue quality and recruitment diversity to scaling digital pathology and harmonizing data across institutions. Lessons from fields like neuroimaging show us the value of shared data models and multi-site coordination, which we believe can and should be applied to tissue-based research. We outline concrete strategies for enhancing tissue quality, standardizing practices, and making brain tissue studies more inclusive and scalable. Our aim with this work is to chart a path forward that enables postmortem brain research to contribute more directly and consistently to translational discovery in aging and neurodegeneration.
Hemali Phatnani, PhD
Assistant Professor of Neurological Sciences (in Neurology)
hp2286@cumc.columbia.edu

