Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
August 2014
Axonally Synthesized ATF4 Transmits a Neurodegenerative Signal across Brain Regions
 |
 |
| Ulrich Hengst, PhD | Jimena Baleriola, PhD |
In Alzheimer's disease (AD), pathological changes spread across the central nervous system, leading to the successive functional impairment of specific regions of the brain and consequently progressive cognitive decline. The molecular mechanisms underlying this spread of disease have recently received considerable research interest, as a stop or slow down of the spread might interfere with the disease progression and lengthen the lifespan of AD patients.
In our research, we focused on the signaling pathways triggered within axons in response to locally sensed oligomeric β-amyloid (1-42) peptide. Aβ1-42 accumulation is one of the hallmarks of AD and considered causative for many pathological changes in AD brain. Because axons are the longest parts of neurons, spanning up to several inches and connecting different areas of the brain, we reasoned that intra-axonal signaling events in response to locally sensed Aβ could cause retrograde changes affecting the entire neuron and might be part of the mechanisms underlain the spread of AD.
In our study published in the August 28th issue of Cell, we observed a strong activation of localized protein synthesis within axons when we applied Aβ1-42 locally to axons, and inhibition of intra-axonal translation or retrograde transport of proteins to the cell body completely prevented cell death in response to Aβ1-42. Localized protein synthesis is a mechanism by which neurons react in a spatially confined and temporally acute manner to changes in their environment. For example, during the development of the nervous system intra-axonal protein synthesis is crucial for the establishment of neuronal connections. In contrast, mature axons have long been considered as translationally inactive. We found that axonal application Aβ1-42 changed the composition of the axonal transcriptome through recruitment of a defined set of mRNAs. Among the recruited mRNAs was the transcript of the transcription factor ATF4. ATF4 was locally synthesized in response to local application of Aβ1-42 to axons and retrogradely transported to the neuronal soma where it caused transcriptional changes, including CHOP production, leading to cell death. When we selectively depleted axons of Atf4 mRNA we could prevent Aβ1-42-dependent neurodegeneration both in cultured hippocampal neurons and more importantly in a mouse model for amyloidopathy. This finding identifies intra-axonal ATF4 synthesis as a central component of the signaling pathways transmitting neurodegenerative signals across brain regions. The patho-physiologcal relevance of our findings is further emphasized by the fact that we observed both ATF4 protein and mRNA with increased frequency in axons in those regions of the brain of AD patients that are thought to be especially vulnerable in AD, such as the entorhinal cortex and the subiculum.
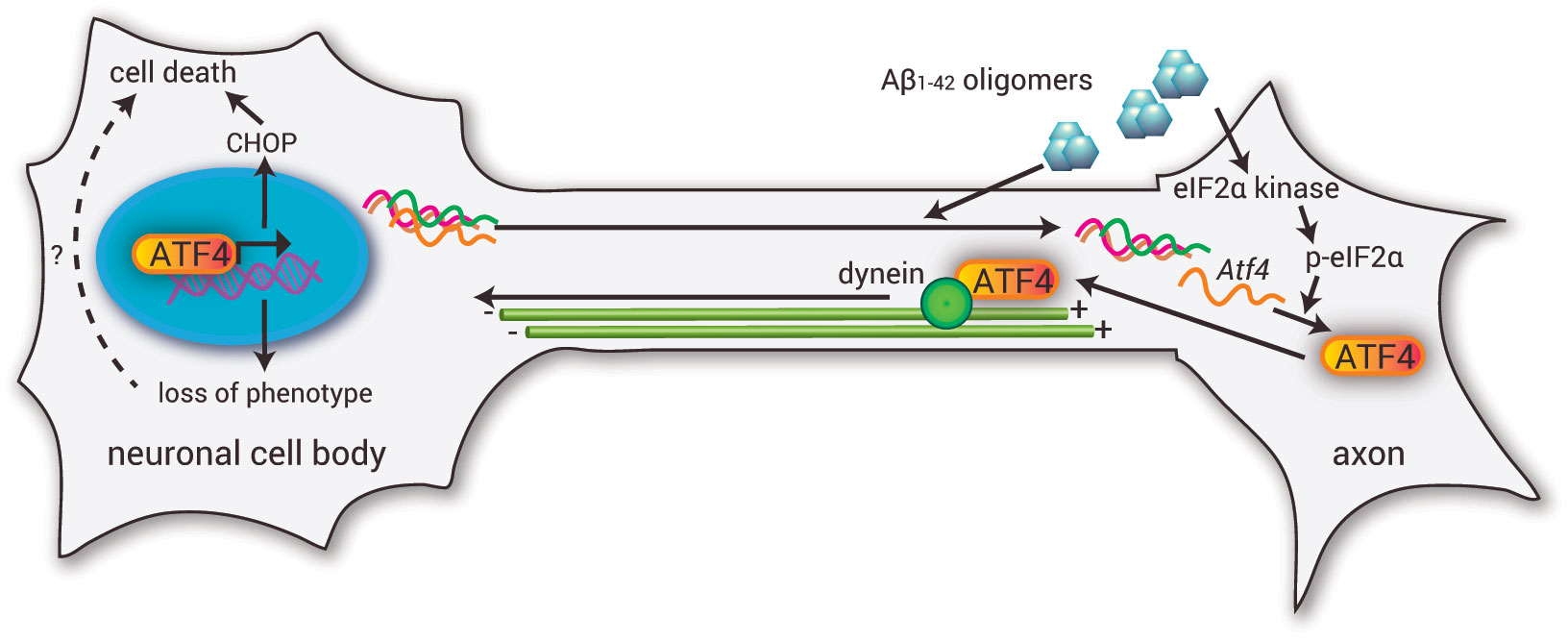 Proposed model: Aβ1-42 oligomers trigger the axonal recruitment and translation of a defined set of mRNAs, including Atf4. ATF4 is synthesized and retrogradely transported to the cell body where ATF4-dependent transcription leads to CHOP-dependent neurodegeneration. |
Our study has several important implications for the understanding of AD. We provide novel mechanistic insight of how Aβ1-42 signaling in the periphery of neurons causes changes in gene expression and eventually triggers neurodegeneration across macroscopic distances. As intra-axonal translation has never been observed in the healthy brain and is likely to occur only at very low levels or not at all, interference with intra-axonal protein synthesis might be a completely new strategy to slow down or prevent the spread of pathology in AD. Additionally, in our axonal transcriptome analysis we identify mRNAs for many AD susceptibility genes that have never before been described as regulated by Aβ. Standard gene expression analysis on the tissue or cellular level is able to detect changes in mRNA abundance but not recruitment to specific subcellular compartments. Our study exemplifies the need to include mRNA transport and other post-transcriptional mechanisms into the investigation of gene expression and provides a new paradigm of how to investigate pathogenic responses in highly polarized cells.
Ongoing research in our group aims to further characterize the transcriptional changes caused by axonally derived ATF4 and we are investigating whether available pharmacological inhibitors of ATF4 synthesis are able to interfere with the spread of pathology in AD brain.
Ulrich Hengst, PhD
uh2112@columbia.edu
Jimena Baleriola, PhD
jb3275@columbia.edu

