Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
August 2016
» #2 LRRK2 and RAB7L1 Coordinately Regulate Axonal Morphology and Lysosome Integrity in Diverse Cellular Contexts
 |  | |
| Karen Duff, PhD | Hongjun (Harry) Fu, PhD |
In a study recently published in the journal PLoS One, a team led by Taub faculty member Dr. Karen Duff has provided evidence that the spread of tau pathology out of entorhinal cortex (EC) into neocortex is associated with neurotoxicity and cognitive impairment in aged EC-tau transgenic mice, which mimics what happens in patients with Alzheimer's disease (AD). It validates the idea that neurotoxicity is associated with the spread of tauopathy. The team's lead scientist, Dr. Hongjun (Harry) Fu, used a variety of sophisticated techniques to study the temporal relationship between tau pathology spread, degeneration, and cognition, which can help define timepoints when therapeutic interventions may be effective.
Fu et al. demonstrated the power of the immunolabeling-enabled three-dimensional imaging of solvent cleared organs (iDISCO) to observe tau pathology in deep structures of the EC-Tau mouse brain, which has greatly facilitated the tracking of tau pathology progression through the mouse brain’s anatomical networks. Using iDISCO, they found several areas affected by tau pathology in older EC-Tau mice that had not been previously identified by 2D immunostaining: the amygdala (AM), piriform cortex (Pir), and anterior olfactory area (AO). Importantly, their findings demonstrate the temporal and spatial relationship between areas as they become affected by tau pathology, including, for the first time in this mouse model, the neocortical areas. As in human AD, the first signs of cognitive impairment correlate with overt tau pathology and cell loss as well as gliosis in the EC, which in turn correlates with the first appearance of tau pathology in the neocortex. Interestingly, they also found that pathological tau was mainly colocalized with neuronal markers, but not microglia or astrocyte markers, although the gliosis is associated with the spread of tau pathology.
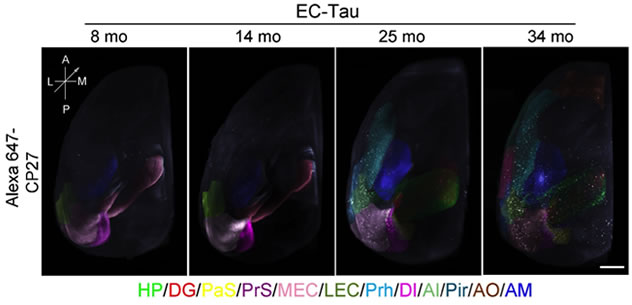 Representative snapshots of 3D images of tau staining in EC-Tau mice using iDISCO. Different brain regions with tau pathology are indicated by superimposed artificial colors. The regions with no or sparse tau immunoreactivity are not colored.
|
These findings, which were also reported by ALZFORUM on August 3rd, help improve understanding of the relationship between the spread of tau pathology, gliosis, and neurodegeneration. Tau pathology and atrophy has been shown in the AM of human AD patients and the Pir, AO and AM have been proposed to play very important roles in olfaction, emotion, and memory in humans. Tau pathology in those areas could also explain the olfactory deficits and psychiatric symptoms seen in patients with early AD. Thus, this EC-Tau mouse model may be a potential model for studying not only cognitive deficits but also olfactory and psychiatric problems seen in patients with early AD.
Karen Duff, PhD
Deputy Director, Taub Institute
Professor of Pathology and Cell Biology (in Psychiatry and in the Taub Institute)
ked2115@cumc.columbia.edu
Hongjun (Harry) Fu, PhD
Associate Research Scientist in the Taub Institute
Hf2296@cumc.columbia.edu
 |  | |
| Asa Abeliovich, MD, PhD | Tomoki Kuwahara, PhD |
Over the past decade, human genetics researchers, including Drs. Lorraine Clark, Karen Marder, and others in the Taub Institute, have made great strides in identifying genes associated with familial or non-familial forms of Parkinson's disease (PD). However, it has been challenging to understand how these genes, or mutations in these genes, play a role in neurodegeneration.
The Abeliovich laboratory has used a variety of model systems to explore the function of several PD-associated genes, including LRRK2. A surprising finding, overall, is that the functions of many of these genes can be linked directly to endosomal intracellular trafficking mechanisms that bring a variety of cargo to the lysosome for degradation. In a recent manuscript, published this month in Scientific Reports, Dr. Abeliovich's team, including first author Dr. Tomoki Kuwahara, generated mice and nematodes deficient in LRRK2 and, in both contexts, found evidence of defective intracellular trafficking to lysosomes, leading to lysosomal abnormalities.
In mice, a surprising aspect is that loss of LRRK2 leads to lysosomal defects, but these are found in kidney proximal tubule cells of older animals, and not in the central nervous system. In pursuit of how LRRK2 may be involved, Kuwahara et al. studied a second PD-associated gene, RAB7L1, and show evidence that the two genes function coordinately in the regulation of intracellular trafficking, both in mice and in nematodes. Furthermore, mice deficient in RAB7L1 look very similar to mice deficient in LRRK2, and the mutations are non-additive. Future work will pursue the relationship between the normal functions of these genes and neurodegeneration in the brain. A key player in this project, which required analysis of kidney pathology, was Dr. Vivette D'Agati in the Department of Pathology and Cell Biology.
Asa Abeliovich, MD, PhD
Associate Professor of Pathology and Cell Biology, and Neurology (in the Taub Institute)
aa900@cumc.columbia.edu
Tomoki Kuwahara, PhD
tkuwahara14@gmail.com

