Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
June 2016
Neuronal Activity Enhances Tau Propagation and Tau Pathology in Vivo
 |  | |
| Karen Duff, PhD | Jessica Wu, PhD |
|
 |  | |
| S. Abid Hussaini, PhD | Gustavo Rodriguez, PhD |
In a study recently published in the journal Nature Neuroscience, a team led by Taub faculty member Dr. Karen Duff has come several steps closer to understanding how neurodegenerative diseases, such as Alzheimer's disease (AD) and Frontotemporal Degeneration (FTD), that are characterized by the accumulation of pathological forms of the microtubule binding protein tau get worse over time. The team's lead scientist, Dr. Jessica Wu (now at MIT), used a variety of sophisticated in vitro cell models to show how the tau protein can spread between brain cells, which is thought to explain how the disease infects new areas of the brain, killing neurons as it goes, and making symptoms worse over time.
Wu et al. demonstrated that cell contact was not needed for the spread of tau between cells, implying that tau passes via the extracellular fluid as it moves from cell to cell, which suggests the mechanism by which tau can spread. Additionally, tau spread was observed using tripartite (3 cell compartment) microfluidic systems. Two team members, Drs. Abid Hussaini and Gustavo Rodriguez, then used complimentary approaches to demonstrate that enhancing neural activity can exacerbate tau pathology in mouse models of FTD, and accelerate the loss of associated cells. Dr. Hussaini applied an optogenetic approach to stimulate brain cells, whereas Dr. Rodriguez used a designer receptor exclusively activated by a designer drug (DREADD) chemogenetic approach, injecting the DREADD drug clozapine-n-oxide (CNO) twice daily to stimulate the neurons. Using these approaches, the investigators found that increased neuronal activity stimulates the release of tau in vitro and enhances tau pathology in vivo.
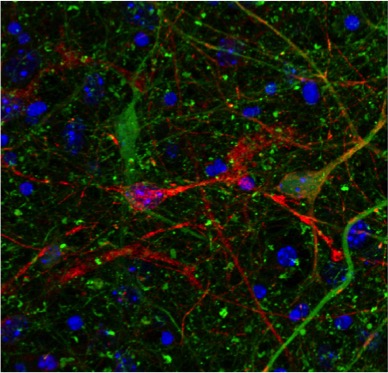
Co-cultured neurons from a mouse expressing mutant human tau (red) or a tau knock-out line expressing GFP in the place of tau (green). Orange cells show where human tau has transferred into tau KO cells.
These findings, which were also reported by ALZFORUM this week, help improve understanding of some of the mechanisms involved in the cell to cell spread of the tau protein in the living brain. Although more work is needed to examine whether the findings are relevant for humans, they do indicate that therapeutic approaches such as deep brain stimulation (DBS), which aims to alter or manipulate brain activity electrically, should be monitored carefully in people with neurodegenerative diseases.
Karen Duff, PhD
Deputy Director, Taub Institute
Professor of Pathology and Cell Biology (in Psychiatry and in the Taub Institute)
ked2115@cumc.columbia.edu
Jessica Wu, PhD
Former Associate Research Scientist in the Taub Institute (now at MIT)
Jinhua07@gmail.com
S. Abid Hussaini, PhD
Assistant Professor of Neurobiology (in Pathology and Cell Biology and the Taub Institute)
sah2149@cumc.columbia.edu
Gustavo Rodriguez, PhD
Postdoctoral Research Scientist in the Taub Institute
gr2501@cumc.columbia.edu
Sleep Disordered Breathing and White Matter Hyperintensities in Community-Dwelling Elders
 |  | |
| Yian Gu, PhD | Sara Rostanski, MD |
Sleep disordered breathing (SDB) is a general term that refers to nocturnal apneas, hypopneas, and intermittent hypoxemia, most often due to obstructive sleep apnea. It is well known that SDB is associated with cardiovascular disease and stroke. However, the extent to which SDB is associated with subclinical cerebrovascular disease is less well understood. Subclinical cerebrovascular disease frequently manifests as white matter hyperintensities (WMH). Recently, Dr. Sara Rostanski, Dr. Yian Gu, and colleagues investigated the association between markers of sleep-disordered breathing (SDB) and white matter hyperintensity (WMH) volume among 483 participants of the Washington Heights-Inwood Columbia Aging Project (WHICAP), a community-based epidemiological study of older adults. High-resolution magnetic resonance imaging (MRI) scans were obtained on a Philips 1.5-Tesla scanner at Columbia University; WMH were derived from standard T2-weighted FLAIR images using an intensity-driven algorithm to provide quantitative measurements of WMH volume. The Medical Outcomes Study-Sleep Scale (MOS-SS) was administered to collect information regarding different aspects of sleep, including sleep initiation and duration, as well as frequency of occurrence of the sleep related event. The study used two MOS-SS questions that measure respiratory dysfunction and snoring during sleep as SDB surrogates. Linear regression models were used to assess the relationship between the two MOS-SS questions that measure respiratory dysfunction during sleep and quantified WMH volume.
Recently published in the journal Sleep, the study found that self-reported SDB was associated with WMH. After adjusting for demographic and vascular risk factors, WMH volumes were larger in individuals with frequent snoring (β = 2.113, P = 0.004) and among those who reported waking short of breath or with headache (β = 1.862, P = 0.048). The findings suggest that the cognitive effects of SDB may be a function of small-vessel cerebrovascular disease, with WMH as a potential mediator. The identification of reversible risk factors such as SDB for WMH has important implications in healthy cognitive aging.
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology and the Taub Institute)
yg2121@cumc.columbia.edu
Sara Rostanski, MD
Postdoctoral Clinical Fellow in the Department of Neurology
skr2140@cumc.columbia.edu

