Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
June 2015
» #2 Connectivity and Circuitry in a Dish Versus in a Brain
SUMO1 Affects Synaptic Function, Spine Density and Memory

Ottavio Arancio, MD, PhD
Post-translational modifications constitute an important step in protein biosynthesis that regulates protein function by attaching functional groups to the amino-acids that compose the protein. This recent Scientific Reports article from the laboratory of Taub faculty member Dr. Ottavio Arancio, in collaboration with the Fraser Laboratory at the Tanz Centre for Research in Neurodegenerative Diseases of the University of Toronto, focuses on protein post-translational modification by Small Ubiquitin-like Modifier-1 (SUMO1). Matsuzaki et al. report the development of a unique transgenic mouse model which has permitted the identification of novel contributions of SUMO1 to synaptic transmission and the stability of dendritic spines. These changes in neuronal form and function also translate into a previously unrecognized significant impairment in learning and memory. To support the mechanism of action underlying these changes, Matsuzaki et al. have identified a number of previously unrecognized SUMO1 conjugation candidates that have an impact on a variety of cellular pathways. These SUMO1 targets are linked to presynaptic function such as a protein called synaptotagmin, which leads to diminished neurotransmitter release. Another target, the cytoplasmic FMR1-interacting protein 1 (Cyfip1), regulates actin organization at synapses and is linked to autism as well as schizophrenia, suggesting a potentially broader impact of SUMO1 modifications on disease and development. The increase in SUMO1 also causes a substantial reduction in neuronal spine density and strength of cell to cell communication. The changes in neuronal function and morphology were also associated with a specific impairment in learning and memory while other behavioral features remained unchanged. These findings point to a significant contribution of SUMO1 modification on neuronal function, which may have implications for mechanisms involved in intellectual disability and neurodegeneration. As such, these results will have a large impact on the neuroscience field.
Previously, the Arancio Laboratory discovered that SUMO2 conjugation, the other type of SUMO conjugation, is reduced in brain tissue from Alzheimer's Disease (AD) patients. While neuronal activation normally induced up-regulation of SUMO2 conjugation, this effect was impaired by amyloid-beta, a toxic protein that is known to play a major role in AD. Amyloid-beta is also a known potent disruptor of synaptic function. Thus, taken together, these studies reveal a unique mechanism for the two types of SUMO, in which SUMO1 and 2 play opposite roles in cell to cell communication and memory. SUMO1 shows a negative impact on synaptic development/stability/plasticity/memory while SUMO2/3 is beneficial to these features.
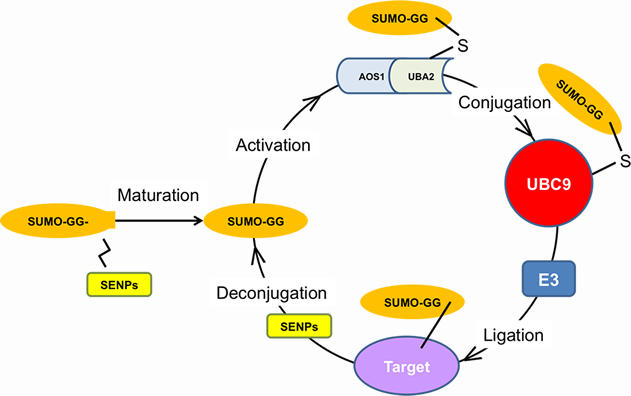 FIGURE LEGEND: The SUMOylation Pathway. The SUMO enzymatic cascade catalyzes the dynamic post-translational modification process of transfer of SUMO protein to other proteins (SUMOylation). The Small Ubiquitin-related Modifier, SUMO is conjugated to its substrates through three sequential enzymatic steps: activation, involving the E1 enzyme, AOS1/UBA2; conjugation, involving the E2 enzyme, UBC9; substrate modification, through the cooperation of the protein ligases, E2 and E3. De-conjugation (De-SUMOylation) occurs through enzymes called SENPs, which recycle free SUMO and unmodified target proteins. The Arancio lab has demonstrated that SUMO1 conjugation negatively affects synaptic morphology, neurotransmission and memory, whereas SUMO2 conjugation positively affects these features. This work gives the impetus to the development of drugs acting at different steps of the pathway to alleviate Alzheimer's disease symptomatology.
|
Ottavio Arancio, MD, PhD
Oa1@columbia.edu
Connectivity and Circuitry in a Dish Versus in a Brain

Ottavio Arancio, MD, PhD
The complexity of the human central nervous system and its inaccessibility to direct studies make its modeling necessary in order to investigate the occurrence of physiological and pathological processes. Because of many limitations in animal disease models, in vitro neuronal models using patient-derived stem cells are currently being developed by numerous laboratories. However, this technological development has led to the need to confirm functionality of the newly generated neurons, with respect to electrical properties of individual neurons, their ability to express pathology, and their capacity to functionally integrate into the brain's circuitry. Additional problems of stem cell derived models include the survival of implanted neurons, the directed differentiation into specific cell types and the tumorigenic potential of incompletely differentiated neurons.
 Figure 1: Immunofluorescence analyses of iPSC-derived neurons. Images by Andrew Sproul of the Taub Institute.
|
The laboratory of Taub faculty member Dr. Ottavio Arancio is at the forefront of research aimed at developing suitable neurons obtained with stem cell techniques. This is particularly true for cells mimicking diseases in which neurons have to be fully mature, such as those in patients with Alzheimer's disease. Dr. Arancio and colleagues recently published a review of studies from numerous laboratories, including the Arancio Laboratory, in the journal of Alzheimer's Research & Therapy. Their conclusion was that recapitulation of human neurological disease following stem cell-derived neuronal differentiation in vitro remains a significant scientific challenge. Nevertheless, the potential of discovering a therapy for numerous diseases awaiting a cure make it one worth pursuing, particularly for neurodegenerative diseases. To this end, maturation protocols that provide stem cell derived neuronal models containing genetic susceptibility in parallel with aging-related factors must continue to be developed in order to accurately model late-onset disorders.
Ottavio Arancio, MD, PhD
Oa1@columbia.edu

