Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
Best Poster Presentations
Taub Institute Retreat October 2014
by Amyloid beta
pyramidal neuron subpopulations
Pathogenic Role of Formin-Mediated Stable Detyrosinated Microtubule Induction by Amyloid beta
Feng Ning Yuan1, Xiaoyi Qu1, Carlo Corona1, Silvia Pasini1, Xinglong Wang2, Roger Lefort1, Michael Shelanski1, and Francesca Bartolini1
1 Department of Pathology & Cell Biology, Columbia University
2 Department of Pathology, Case Western Reserve University
Francesca Bartolini and Richard Mayeux
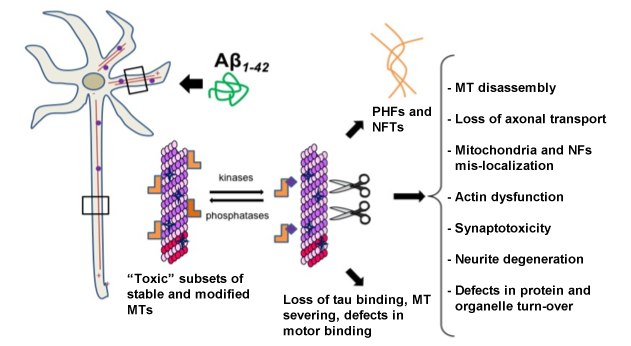
The molecular nature of the link between Aβ and hyperphosphorylated tau remains a subject of great debate. Our novel hypothesis is that Aβ induces "toxic" post-translationally modified microtubules through the activation of specific receptor-mediated pathways, and that chronic exposure to Aβ triggers deleterious increases in tau hyperphosphorylation in an attempt to maintain appropriate levels of unmodified microtubules. In support of our hypothesis, we have preliminary evidence that detyrosinated tubulin is upregulated in the hippocampi of animal models of AD and in AD patients. Importantly, we found that oligomeric Aβ1-42 induces detyrosinated microtubules in neurons independently of tau expression and abrogation of this activity by formin inhibition is sufficient to prevent tau hyperphosphorylation, axonal traffic damage, and synaptotoxicity by Aβ. Our studies suggest a pathogenic role for modified microtubules in AD and explore the significance of a new class of cytoskeletal regulators as potential therapeutical targets to rescue loss of neuronal function and cognition in AD.
 |
Francesca Bartolini, PhD
Assistant Professor of Pathology and Cell Biology, CUMC
fb2131@cumc.columbia.edu
Differential Responsiveness to Entorhinal Cortical Input Distinguishes CA1 Pyramidal Neuron Subpopulations
Arjun V. Masurkar1,2, Daniel Lowes3, Steven A. Siegelbaum4
1Division of Aging and Dementia, Department of Neurology, Columbia University
2Department of Psychiatry, Columbia University
3Oberlin College, Amgen Scholars Program at Columbia University
4Department of Neuroscience, Columbia University

Gil DiPaolo and Arjun Marsurkar
Area CA1 is the first region of the hippocampus affected in early stage Alzheimer’s disease (AD) with regard to neurofibrillary tangles within pyramidal cells. However, this pathology is not uniform on a cell-to-cell level. The mechanism behind this selective vulnerability is unknown, but may shed light on AD pathogenesis. We aimed to study the physiological heterogeneity of CA1 pyramidal cells in the non-diseased state, hypothesizing that differences may relate to such selective vulnerability to disease. Using the in vitro mouse hippocampus slice preparation and whole cell recording, we found that in mid-CA1, the pyramidal cells, divided into superficial and deep populations (sPCs, dPCs), have similar intrinsic properties and are excited to the same extent by CA3 input onto the proximal dendrites. Strikingly, with stimulation of entorhinal cortical (EC) input onto the distal dendrites, sPCs showed excitatory responses nearly three times larger than those in dPCs. We then used pharmacology, quantitative morphometry, and computational modeling to show that this was not due to differences in intrinsic ion currents or dendritic morphology, but more likely due to strength of EC connectivity. In support of this, we also found preliminary evidence that sPCs are more responsive to EC in the majority of CA1, but that the differential responsiveness may change in concordance with a shift in which part of EC is giving the input. In sum, this supports that a primary component of CA1 pyramidal cell heterogeneity is direct cortical drive, and that sPCs comprise a subpopulation that are thus hyperactive in the majority of CA1. Current work is aimed at further supporting this hypothesis as well as testing if this hyperactivity confers selectivity vulnerability in normal aging and AD through the use of mouse models.
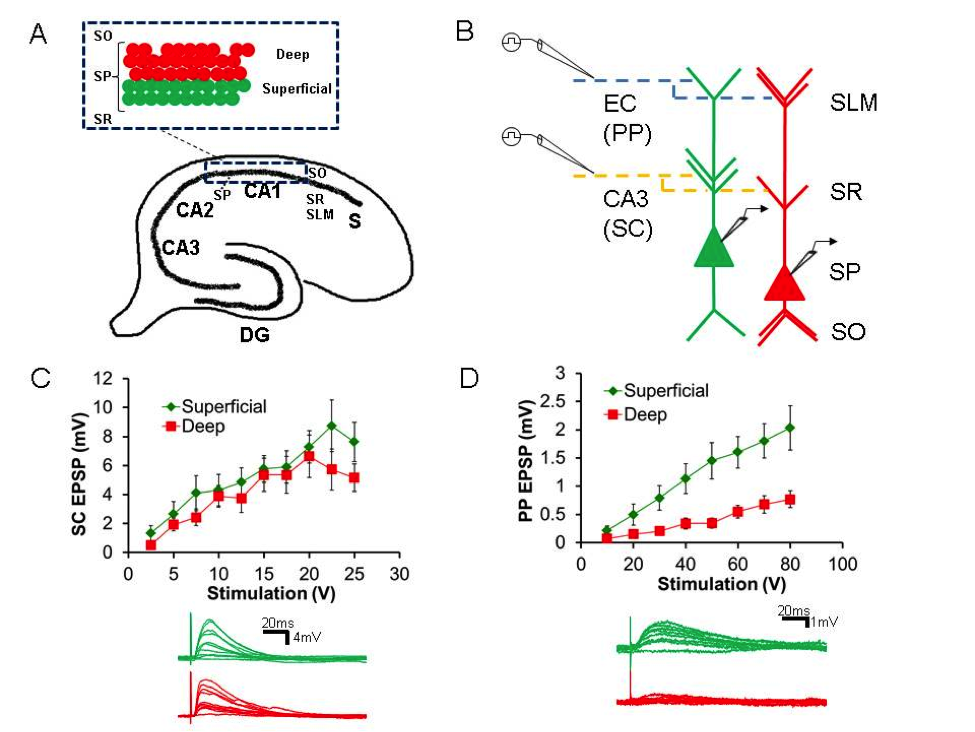 A. Diagram of hippocampal formation with (inset) delineation of deep (dPCs) and superficial (sPCs) pyramidal cell subpopulations B. Schematic of slice experiment to examine responsiveness of sPCs (green) and dPCs (red) to EC and CA3 input C. Above: Stimulus-responsive curves to CA3 (SC) input are similar. Below: Sample traces D. Above: Stimulus-response curves to EC (PP) input show increased responsiveness in sPCs. Below: Sample traces.
|
Arjun V. Masurkar, MD, PhD
Fellow, Division of Aging & Dementia, Department of Neurology and Department of Psychiatry, CUMC
avm2114@columbia.edu

