Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
Best Poster Presentations
Taub Institute Retreat October 2015
DREADDs Activation in the Medial Entorhinal Cortex (MEC) of EC-Tau Mice
Rodriguez GA 1,2, Hussaini SA1,2, and Duff KE 1,2.
1Taub Institute for Research on Alzheimer's disease and the Aging Brain, Columbia University, New York, NY
2Department of Pathology and Cell Biology, Columbia University, New York, NY
Gustavo A. Rodriguez, PhD
The entorhinal cortex (EC) is one of the first structures to exhibit neurofibrillary tau tangles in Alzheimer's disease (AD), as well as significant neurodegeneration as the disease progresses. The gradual accumulation of pathological tau in AD may lead to impaired firing properties of EC neurons, and eventually, to network dysfunction in the entorhinal-hippocampal circuit, facilitating tau spread. To investigate the relationship between neuronal activity and in vivo tau spread, we collected single-unit recordings and local field potentials in the medial entorhinal cortex (MEC) of awake, behaving transgenic mice that preferentially express pathological tau in the EC (EC-Tau mice). Moreover, we implemented a chemogenetic system (DREADDs; Designer Receptor Exclusively Activated by Designer Drugs) wherein spatiotemporal control over neuronal activity can be regulated. Time-course and dose response experiments were performed using two different versions of the DREADDs receptors that either increase or suppress firing in excitatory MEC neurons. We found that 5-10MG/KG Clozapine-N-Oxide (CNO) was sufficient to significantly increase or suppress total spiking activity for over 60 min in EC-Tau mice, with onset of DREADDs activation occurring at 15-20 min post-CNO administration. Single-unit analyses revealed a heterogeneous population of MEC Layer II/III neurons that responded either 1.) sharply to CNO, with a peak activation window occurring at ~20 min and lasting 10-15 min, 2.) broadly to CNO, with activation onset beginning at ~20 min post-drug admin and remaining level, or 3.) did not respond at all. Single-unit analyses also revealed a population of spatially modulated MEC cells, known as grid cells and border cells, that respond well to DREADDs activation. Using this model, we can now address whether chronic activation or suppression of MEC activity can facilitate or delay pathological tau spread in vivo.
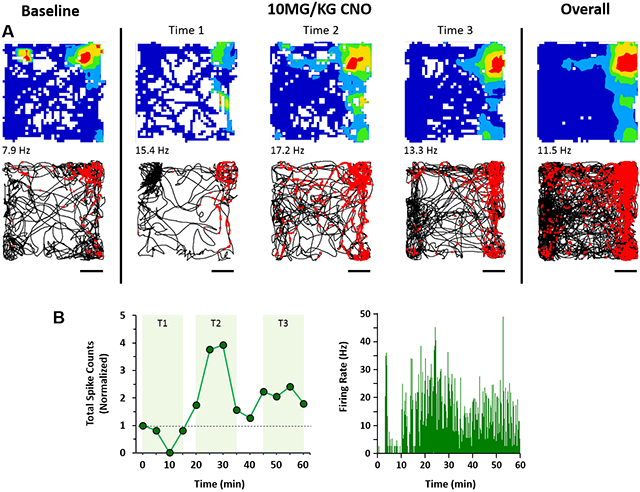 DREADDs activation in a putative MEC Border Cell. EC-Tau mice expressing hM3Dq DREADDs in excitatory cells of the MEC were administered CNO and allowed to explore an open field during electrophysiological recording. A. Top panels, spatial firing patterns of a putative border cell during a 15-min baseline recording session, during three time windows after DREADDs activation, and the overall firing pattern. The color scale for the firing rate map is from 0 Hz (blue) to peak firing rate (red). Spiking activity for the cell is overlaid onto the trajectory of the mouse (black lines) during the sessions. Scale bar: 100mm. B. Total spike count and spike density plots show a sharp activation window for the MEC border cell due to DREADDs activation.
|
Gustavo A. Rodriguez, PhD
Postdoctoral Research Scientist in the Taub Institute
Laboratory of Karen Duff, PhD
gr2501@cumc.columbia.edu
Amyloid Precursor Protein (APP) is Ubiquitinated at Multiple Sites in the COOH-Terminal Domain as a Signal for Endosomal Sorting
Williamson RL 1,2, Chamoun Z 1,2, Morel E 3, Small SA 1, and Di Paolo G 1,2.
1 Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Columbia University, New York, NY
2 Department of Pathology and Cell Biology, Columbia University, New York, NY
3 Institut Necker Enfants-Malades, Université Paris Descartes, Paris, France
Rebecca L. Williamson
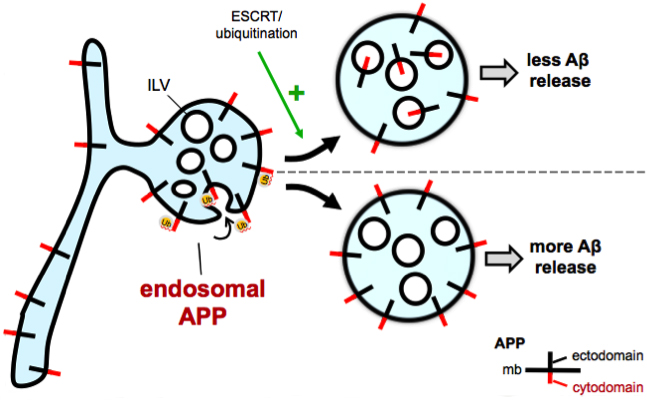
Amyloid plaques, a neuropathological hallmark of Alzheimer's disease (AD) are largely composed of amyloid beta (Aβ) peptide, derived from cleavage of its parent protein, amyloid precursor protein (APP). Though the subcellular locations where APP, a type I transmembrane protein, processing occurs remain poorly understood, growing evidence suggests that amyloidogenic cleavage occurs in endosomes. Our lab implicated an endosomal sorting pathway for APP that has major consequences for its amyloidogenic processing. The molecular basis involves recognition of ubiquitinated APP by components of ESCRT (endosomal sorting complex required for transport) to sort APP from the limiting membrane of endosomes into intraluminal vesicles (ILVs) for eventual lysosomal degradation. The five lysine residues in the APP C-terminal domain are potential sites of ubiquitination and might act as signals for APP trafficking and processing within the cell. Lysine-to-arginine mutations in the C-terminal domain are deficient in ubiquitination, lead to mislocalization of APP from the endosome interior to the limiting membrane, and increase levels of Aβ. We hypothesize that mutation of lysine residues of APP leads to endosomal mislocalization by disrupting ESCRT recognition. We speculate that, instead of being degraded in lysosomes, APP builds up on the endosome limiting membrane where it is processed by BACE1 and γ- secretase. Indeed, chemically-induced heterodimerization of APP and ubiquitin rescues the increase in Aβ seen in these APP ubiquitin-deficient mutants. More work is necessary to determine whether these changes in APP metabolism can be recapitulated in vivo and whether elements of this ubiquitination pathway can be harnessed to affect Aβ generation and plaque deposition.
 |
Rebecca L. Williamson
Graduate Student, Columbia Doctoral Program in Neurobiology and Behavior
Laboratory of Gil Di Paolo, PhD
rlw2159@cumc.columbia.edu

