Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
September 2014
» A Time Course Analysis of the Electrophysiological Properties of Neurons Differentiated from Human Induced Pluripotent Stem Cells (iPSCs)

Adam M. Brickman, PhD
Over the past several years, we have been studying the contribution of white matter hyperintensities (WMH), areas of increased signal abnormalities visualized on T2-weighted MRI scans (see Figure 1), to the risk, progression, and symptom manifestation of Alzheimer's disease (AD). Our previous work showed that older individuals with high amounts of WMH distributed in posterior brain regions are at particular risk for developing the disease and that these lesions appear to interact with other biological markers of AD. In an attempt to understand the mechanisms underlying the association between white matter abnormalities and AD, we have begun a series of studies that systematically examines histopathological markers in the white matter tissue of individuals with and without AD.
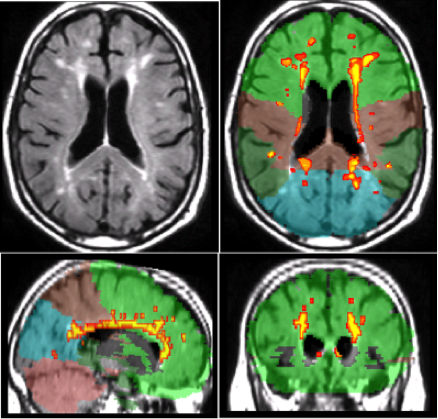
Figure 1. White matter hyperintensities seen as increased signal on T2-weighte MRI scan (upper left panel) and labeled by lobe (other three panels).
Despite a growing recognition of the importance of white matter abnormalities in the pathogenesis of AD, the pathological basis of white matter degeneration remains to be elucidated. While multiple studies attributed white matter abnormalities to concomitant small vessel disease or Wallerian-like degeneration, others suggest that primary white matter pathology may be due, at least in part, to other mechanisms, including toxic Aβ peptides. Recently published in Acta Neuropathologica Communications, our study investigated this possibility further by focusing on levels of soluble Aβ-40 and Aβ-42 peptides in postmortem cerebral white matter from AD patients and non-AD controls. We elected to measure soluble amyloid levels as, in recent years, extensive evidence has accumulated to suggest that it is the levels of soluble Aβ oligomers, rather than insoluble Aβ fibrils, that correlate with the extent of synaptic loss and the severity of cognitive impairment in AD.
Twenty-two cases from the New York Brain Bank at Columbia University were included in the study; 12 were diagnosed pathologically with AD and 10 were non-AD controls. Fresh frozen blocks from Brodmann areas 1, 2, and 3 (primary somatosensory cortex) and BA9 (dorsolateral prefrontal cortex) were used to parallel our in vivo neuroimaging studies. White matter was manually dissected from each block and Aβ-40 and Aβ-42 levels were measured with high sensitivity ELISA.
We compared white matter Aβ-40 and Aβ-42 across the diagnostic groups (AD vs. control) and region (anterior vs. posterior). Alzheimer's patients had higher average white matter Aβ-40 and Aβ-42 levels than controls and there was a trend for Aβ-42 levels to be higher in anterior regions. These differences persisted even after we controlled statistically for measures of cortical amyloid pathology.
The findings are consistent with the hypothesis that white matter damage in AD could be due, in part, to the toxicity of Aβ peptides. We look forward to following-up the work by examining how other histopathological markers in the white matter interact.
Adam M. Brickman
amb2139@cumc.columbia.edu
James E. Goldman
Jeg5@cumc.columbia.edu
Lyndsey E. Collins-Praino
lyndsey.collins-praino@adelaide.edu.au

Ottavio Arancio, MD, PhD
Use of stem cells is likely to provide a powerful tool for the fight against neurodegenerative diseases. In the last decade, many studies have been published demonstrating the possibility to generate functional neurons from mouse and human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). The ultimate goal of these studies is to establish in vitro models of the diseases in order to investigate their etiopathogenesis and to test potential therapeutics. To this end, a fundamental question remains to be addressed: How reliably do these IPSC derived preparations phenocopy the diseases? To answer it, in a recent study published in PLOS ONE, we characterized the evolution of several electrical properties of human iPSC-derived neurons generated with a widely used protocol, including the resting membrane potential, the generation of action potentials, and the formation of synaptic activity.
As expected, we found that during cellular maturation the resting membrane potential became more negative, the expression of voltage-gated sodium channels increased to the point that neurons might become capable of firing action potentials. Moreover, the percentage of cells exhibiting synaptic activity, as demonstrated by the presence of miniature excitatory post-synaptic currents (mEPSCs), increased over time. These changes were associated with an increase of intracellular Ca2+ levels, a key ion involved in several vital enzymatic reactions. We also demonstrated that co-culturing iPSC-derived neurons with mouse glial cells accelerated the development of the electrical parameters as compared to pure iPSC-derived neuronal cultures.
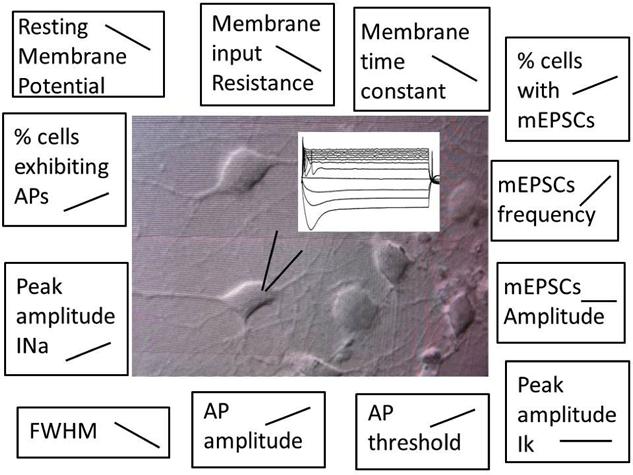 This schematic shows a patch clamp recording of action potentials on an iPSC-derived neuron. The parameters studied in our work and their evolution over time are reported on the edge of the picture.
|
In conclusion, we observed that the maturation of the neurons is not uniform, since some of them are able to fire after few days and others do not respond to a depolarization after 55 days of maturation, and even after 55 days of culture the majority of the cells do not show mEPSCs, demonstrating a slow and somehow incomplete maturation of the neurons in culture. In summary, the pathophysiological relevance of studies performed from neurons derived from stem cell technology should be properly evaluated by taking into account the electrical status of the newly generated neurons. Moreover, our findings help establish baseline conditions for comparisons in future studies using neurons derived from stem cells, both in basic and translational research of neurologic disorders.
Ottavio Arancio
Oa1@cumc.columbia.edu
Deborah Pre
deborah.pre@unipv.it

