Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Roger Lefort, PhD
 Roger Lefort, PhD
Roger Lefort, PhDAt its core, Alzheimer's disease (AD) is a disease of synapses. Indeed, synaptic dysfunction and the loss of synapses are invariable early occurrences in patients, leading to an impairment of cognitive abilities and amnestic symptoms. Consequently, correcting this loss represents a viable and arguably requisite therapeutic strategy, especially in the early stages of the disease.
Elucidating the precise molecular nature of synapse loss in AD has been the focus of our research. Our research has implicated the Rho-family GTPases, RhoA and Rac1, as key mediators of the synaptotoxic effects of Abeta in neurons. RhoA and Rac1 play critical roles in regulating dendritic spine dynamics by controlling the actin cytoskeleton. RhoA and Rac1 have antagonistic effects: Rac1 favors the formation and stabilization of new spines, whereas RhoA blocks their sprouting and promotes their destabilization. This implies that an imbalance in RhoA/Rac1 signaling may have deleterious effects on dendritic spine maintenance. Consistent with this idea, our studies show that spine loss in neurons exposed to Aβ correlates with increased RhoA and decreased Rac1 activity (Figure 1).
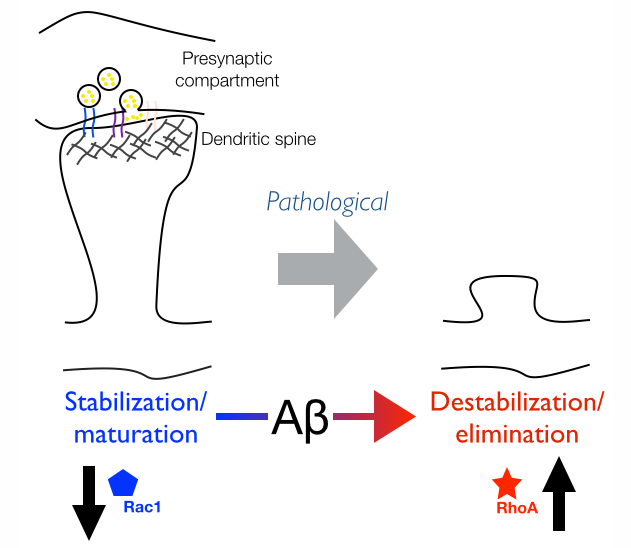 Figure 1 | Aberrant Rho-GTPase signaling in AD leads to loss of dendritic spines.
|
Moreover, blocking RhoA activity neurons completely abrogates the synaptotoxic effects of Aβ suggesting that RhoA may be a therapeutic target for AD, a strategy we are currently exploring (Figure 2). We've also been particularly interested in understanding the nano-mechanical properties of synapses, an often-overlooked contributor to their normal function. In collaboration with Dr. Ozgur Sahin (Biological Sciences Department, Columbia University) we've been studying the specific role of mechanics in synaptic structure and function and investigating how mechanics are altered in AD to contribute to synaptic dysfunction and memory loss.
Another area of interest has been to characterize receptors that play important roles in Abeta signaling, leading to neurodegeneration and/or synaptic dysfunction, with a specific focus on Ephrin receptors (EphR), a large family (16 receptors) of widely expressed receptor tyrosine kinases. Interestingly, recent genome-wide association studies (GWAS) have identified one family member, EphA1, as a risk factor for late-onset AD, through a mechanism that has yet to be explored. In collaboration with Drs. Richard Mayeux and Badri Vardarajan of the Taub Institute, we’ve been actively investigating how EphA1 contributes to AD pathogenesis.
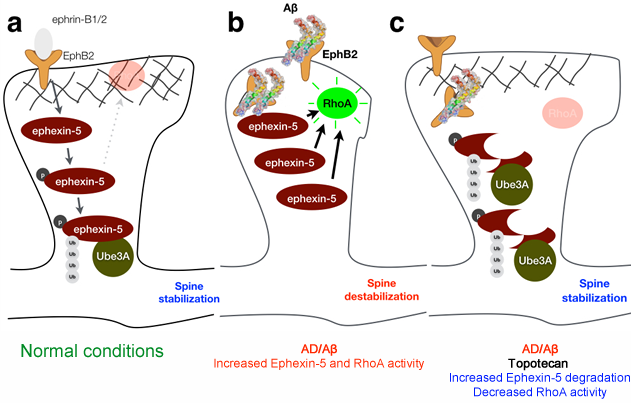 Figure 2 | Proposed model for indirect modulation of RhoA activity. (a) Under physiological conditions, RhoA activity is attenuated due to proteasomal degradation of Ephexin-5, mediated by Ube3A. (b) In the presence of Abeta, Ephexin-5 levels accumulate in the dendritic spines, leading to increase RhoA activity and spine collapse. (c) Increasing Ube3A levels promotes the degradation of Ephexin-5, and abrogates the increase in RhoA activity, thereby preventing dendritic spine collapse.
|
To address these questions, our lab utilizes a wide array of multidisciplinary tools, including cell biological techniques, imaging, fluorescence and torsional harmonic atomic force microscopy, electrophysiology and biochemistry in both neuronal cultures (primary and iPSC-derived) and in rodent models of AD.
 Members of the Lefort Laboratory include, from left to right, Pauline Escot, Hardik Patel, Roger Lefort (PI), and Peter Tzelios. Not pictured: Pablo Robador and Yoon Kim.
|
Current projects in the lab include:
Pablo Robador, a post-doctoral research scientist, is testing our hypothesis that modulating RhoA activity can be a viable therapeutic strategy for AD. This is accomplished indirectly by promoting the proteasome-mediated degradation of a key RhoA activator, Ephexin-5, whose activity is increased by Aβ.
Pauline Escot is a visiting graduate student from France who’s been investigating several pathways that regulate the degradation of Ephexin-5.
Yoon Kim, a 1st year graduate student in the Department of Pathology and Cell Biology, has been investigating the functional consequences of several deleterious mutations in EphA1 (identified by Drs. Richard Mayeux and Badri Vardarajan) and how they contribute to AD pathogenesis.
Hardik Patel is a research assistant, who’s been investigating a previously unknown physiological function of EphA1 in cell proliferation.

