Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Gunnar Hargus, MD, PhD

Gunnar Hargus, MD, PhD
After completion of my residency and fellowship training in anatomical pathology and neuropathology in the Department of Pathology and Cell Biology at Columbia University, I started my research lab in the Taub Institute. My research focuses on human pluripotent stem cells and their application in developmental biology, regenerative medicine, and in modeling of neurodegenerative diseases, with a special focus on frontotemporal dementia (FTD).
FTD is a heterogeneous group of neurodegenerative dementias that is characterized by atrophy of the frontal and temporal lobes leading to impairment of behavior, language and cognition. FTD can be caused by mutations in the gene encoding the microtubule-associated protein tau (MAPT) resulting in widespread deposition of hyperphosphorylated tau protein in neurons and glial cells in the brains of patients. Before coming to Columbia, I worked at the Max Planck Institute for Molecular Biomedicine in M├╝nster, Germany, where we generated induced pluripotent stem (iPS) cells from FTD patients carrying the MAPT N279K mutation and isogenic, gene-corrected control cells using CRISPR/CAS9 editing technology. These FTD and control cells were differentiated into neurons, astrocytes, and oligodendrocytes using optimized differentiation protocols. These studies demonstrated pronounced tau pathology, impaired microtubule function, an increased susceptibility to oxidative and endoplasmic reticulum stress, as well as disturbed whole transcriptome profiles in FTD cells.
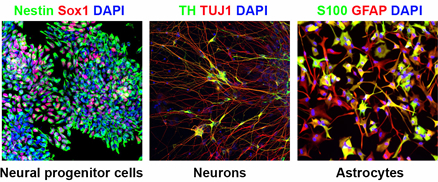
Figure 1. In vitro differentiation of human iPS cells. By using optimized differentiation protocols, human iPS cells can efficiently be differentiated into neural progenitor cells, neurons and astrocytes.
Current studies within my lab focus on elucidating the mechanisms of disturbed metabolic function in FTD neurons, which display increased oxygen consumption and ATP production compared to their isogenic controls. Assays for this study include the 'seahorse assay', cell toxicity studies, and mass spectrometry. Another ongoing project involves the transplantation of FTD and control iPS cell-derived neural cells into the brains of immunocompromised mice to assess survival, migration, and neural integration of these cells in a physiologic microenvironment in vivo. This project also includes an analysis of astrocyte-mediated effects on neuronal survival in FTD, which we study in neuron-astrocyte co-cultures in vitro and in mixed transplants. Recently, our lab received support to determine disease phenotypes in FTD-iPS cell-derived neurons and astrocytes at a single cell level in vitro and after intracranial transplantation in mice by applying single cell RNA sequencing. We also started a project to determine cell type-specific gene expression profiles in postmortem brains of FTD patients with the MAPT N279K mutation using single-nucleus RNA sequencing. For different aspects of our studies, we work closely with Drs. Markus Siegelin, Ismael Santa-Maria Perez, Peter Canoll, and Jim Goldman in the Department of Pathology and Cell Biology, as well as Drs. Philip L. De Jager and Vilas Menon in the Department of Neurology and Taub Institute at Columbia University.
 From left to right: Visiting students Brandon Graser and Minna Jaskari, Gunnar Hargus and postdoctoral research fellow Tristan Winters.
|
Gunnar Hargus, MD, PhD
Assistant Professor of Pathology and Cell Biology
gh2374@cumc.columbia.edu

