Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Clarissa Waites, PhD
 Clarissa Waites, PhD
Clarissa Waites, PhDThe goal of our research is to elucidate the mechanisms of protein turnover and degradation in the nervous system. Neurons rely on cellular degradative pathways (e.g. autophagy, ubiquitin proteasome system) to eliminate damaged or misfolded proteins and maintain protein homeostasis. Neuronal synapses, in particular, require efficient mechanisms for protein degradation, as damaged proteins jeopardize the precisely regulated molecular interactions essential for synaptic transmission. Indeed, dysfunction of degradative pathways is associated with synaptic failure and neurodegenerative disease, including Alzheimer's, Huntington's, and Parkinson's diseases. However, while the importance of protein degradation for nervous system health is widely recognized, very little is known about the basic mechanisms that regulate protein degradation in neurons and at synapses, in particular.
Our laboratory is investigating the molecular pathways responsible for protein turnover and degradation at the synapse. We want to understand how these pathways function in healthy neurons, and how they are perturbed under pathological conditions such as neurodegenerative disease. These studies will identify molecules that promote or inhibit protein degradation under different conditions, and may therefore be useful therapeutic targets for the treatment of neurodegenerative disease.
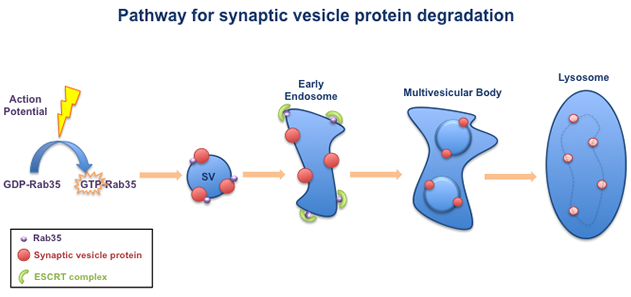 Our lab has identified a pathway that is required for the degradation of synaptic vesicle (SV) proteins. This pathway is turned on by neuronal activity, in the form of electrical impulses called 'action potentials'. Action potentials switch on the small GTPase Rab35, which is associated with synaptic vesicles. Activated Rab35 in turn recruits a group of molecules called the ESCRT complex. The ESCRT complex packages the SV proteins into multivesicular bodies, which are transported to lysosomes for degradation.
|
Another focus of our research is on protein ubiquitination, a post-translational modification that targets proteins for degradation but also has non-degradative functions such as coordinating protein trafficking. At synapses, "regulated" ubiquitination is implicated in synapse formation/stabilization, neurotransmitter release, and plasticity mechanisms, while "dysregulated" ubiquitination is linked to synapse destabilization/loss and neurodegenerative disease. We are, therefore, interested both in how ubiquitination regulates normal synaptic function, and in how aberrant ubiquitination disrupts protein homeostasis and contributes to neurodegeneration.
For our studies, we use gain- and loss-of-function manipulations (including shRNA knockdown and CRISPR/Cas9 gene editing) combined with live imaging, electron and super-resolution microscopy, electrophysiology, and biochemistry in primary neuronal cultures and in rodent models of neurodegenerative disease.
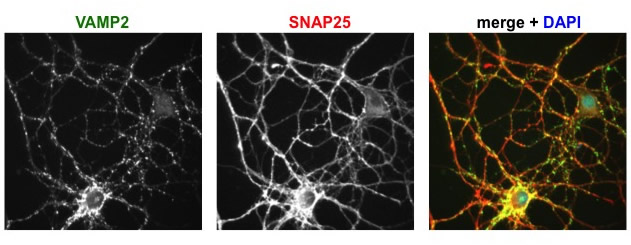 Immunofluorescence images of cultured hippocampal neurons, showing the distributions of two proteins studied in the lab. VAMP2 is found on synaptic vesicles, SNAP25 on the plasma membrane, and DAPI labels nuclei.
|
Current projects in the lab include:
Patty Sheehan, a graduate student in Pathobiology and Molecular Medicine, has identified an essential molecular pathway for the degradation of synaptic vesicle proteins. She is currently investigating whether this pathway mediates the degradation of other classes of synaptic proteins (e.g. cell-surface receptors), and whether it is activated in response to pathological conditions such as oxidative stress or protein aggregation.
Anne Beskow, a postdoctoral fellow, is examining how ubiquitination contributes to the degradation of synaptic vesicle proteins. In particular, she is investigating the role of a novel membrane-associated ubiquitinating enzyme in this process. Anne is also leading a high-throughput screening project to identify novel fluorescent probes that label synapses. The goal of this project, performed in collaboration with Dr. Dalibor Sames (Dept. of Chemistry) and the Columbia Genome Center HTS facility, is to develop probes for non-invasive imaging of synapses in the living human brain, thereby facilitating the diagnosis and treatment of neurodegenerative diseases.
 Members of the Waites Laboratory include, from left to right, Giuseppe Cortese, Eleanor Churchland (lab mascot), Clarissa Waites (PI), Patty Sheehan, and Anne Beskow. Not pictured: Cyndel Vollmer and JoĆ£o Luis Vaz Lima da Silva.
|
JoĆ£o Luis Vaz Lima da Silva, an MD/PhD student in the joint program between Columbia University and University of Minho (Portugal), is investigating the role of several small Rab GTPases in the trafficking and degradation of amyloid precursor protein in neurons. These studies will help us evaluate whether Rabs are viable therapeutic targets for the treatment of Alzheimer's disease.
Giuseppe Cortese, a postdoctoral scientist, is studying the role of Parkin, a ubiquitinating enzyme linked to Parkinson's disease, in glutamatergic synaptic transmission. Giuseppe has pioneered our lab's use of electrophysiological techniques to study synaptic function and dysfunction, in collaboration with the Columbia Stem Cell Electrophysiology Core.
Cyndel Vollmer, a graduate student in the Integrated Program, is investigating the role of protein ubiquitination in neurotransmitter release, using both electrophysiological and biochemical techniques. Her findings, so far, indicate that ubiquitination is an important regulator of the trafficking and activity of synaptic vesicle proteins essential for neurotransmitter release.

