Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Elizabeth M. Bradshaw, PhD

Elizabeth M. Bradshaw, PhD
A number of genetic loci associated with Alzheimer’s disease (AD) and Parkinson’s disease (PD) appear to exert their influence on disease through the innate immune system. Therefore, there is a fundamental need to better understand the molecular function of proteins associated with these complex neurodegenerative diseases, be they genetically associated or identified through other -omics efforts, in innate immune cells, presumably microglia - the innate immune cell of the central nervous system (CNS). Thus, determining the molecular processes that these neurodegenerative disease-associated proteins are manipulating in the innate immune system, and how that impacts susceptibility to AD and PD is a current focus of my laboratory within the Taub Institute. As co-director of basic research in the Center for Translational and Computational Neuroimmunology (CTCN; directed by Taub faculty member Dr. Philip L. De Jager), I lead highly collaborative projects to better understand these innate immune cells in the CNS, and to translate recent “big data” findings to functional outcomes and biological pathways that can be targeted for therapeutic intervention.
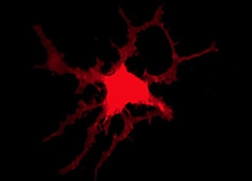
Figure 1. In addition to upregulating microglia defining genes, monocyte-derived microglia-like cells change morphology during differentiation and while quite heterogeneous, many become more reminiscent of homeostatic microglia found in the CNS.
As a clinical immunologist, I work predominately with human model systems, and here is where human microglia present a number of unique challenges. One such challenge is that they are not readily accessible in the same manner as peripheral cells. In order to translate genetic findings, our team needs microglia from a large number of individuals. Therefore, we optimized a protocol to derive microglia-like cells from monocytes (Figure 1), allowing us to 1) identify genotype-driven gene/protein-expression changes, 2) modulate key proteins within these cells to determine which activity of these polyfunctional cells is key to the disease association and 3) easily screen small molecules for therapeutic effect in individuals selected for their genetic background or disease state. The other human immunology tool that I prioritized as a key resource for our work was the ability to isolate viable human microglia from any autopsy center in the continental U.S., allowing our team to probe these critical immune cells in a variety of ways.
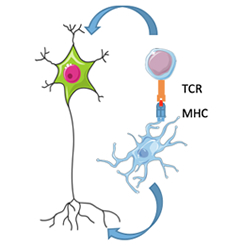
Figure 2. The role of microglia as antigen presenting cells and the influence of both microglia and T cells on neurons in AD and PD is currently not well understood. .
With these tools now in hand, we are mapping the binding partners of the innate immune specific neurodegenerative-associated proteins in microglia, and finding new therapeutic targets, some of which are themselves neurodegenerative-associated proteins. With our ability to make microglia-like cells from large numbers of individuals and validate in ex vivo microglia, we are examining if there is convergence onto certain cellular pathways in microglia for AD and PD. For example, in both AD and PD, major histocompatibility complex (MHC) molecules are genetically associated, suggesting that microglia’s ability to function as antigen-presenting cells in the CNS may play a role in disease susceptibility (Figure 2). In collaboration with Taub faculty member Dr. Wassim Elyaman, I aim to examine the ability of microglia to present antigen to T-cells, and determine how we can manipulate this interaction in a therapeutically beneficial manner.
Elizabeth M. Bradshaw, PhD
Adler Assistant Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Institute for Genomic Medicine)
emb2280@cumc.columbia.edu

