Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Kapil V. Ramachandran, PhD

Kapil V. Ramachandran, PhD
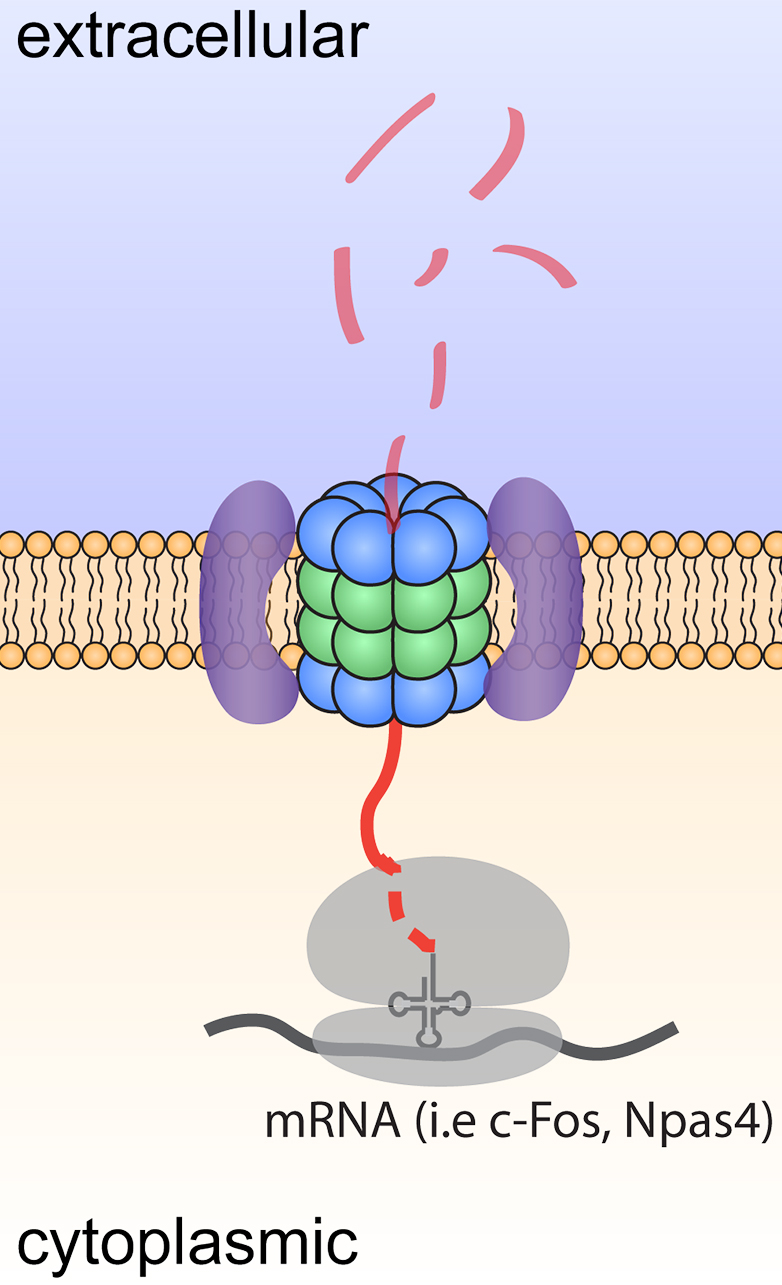
Figure 1. Model depicting degradation and signaling by neuroproteasomes.
Neurons are postmitotic cells that synthesize 2-5 fold more protein than mitotic cells, yet live for decades without accumulating protein aggregates. There is an almost implicit requirement for unique neuronal mechanisms for maintaining proteostasis. Indeed, one of the most penetrant phenotypes that is observed in the both the brain of patients with Alzheimer’s Disease and those who have age-related cognitive decline is the accumulation of protein aggregates. Yet, hardly any neuronal-specific mechanisms of protein homeostasis have been identified.
While many mechanisms exist to control proteostasis, the most dominant mechanism is through the proteasome. Proteasomes are large intracellular multicatalytic molecular machines through which >90% of proteins are degraded. I made the discovery of a neuronal-specific plasma membrane-localized proteasome, or neuroproteasome. Our discovery that neuroproteasomes are uniquely localized to the plasma membrane and degrade only newly synthesized proteins, not ubiquitylated full-length proteins, controverts scientific dogma. Solving how neuroproteasomes contribute to normal neuronal function and how neuroproteasome loss contributes to neurodegeneration and aging provides 1) a novel set of molecular targets to manipulate proteostasis in neurons through neuroproteasomes and 2) evidence for a new mechanism for age-related cellular decline and selective neuronal vulnerability. We would argue that nascent chains are particularly susceptible to misfolding and aggregation, positioning the NMP as a potentially potent and critical mediator of neuronal proteostasis.
Equally intriguingly, these transmembrane-like neuroproteasomes degrade substrates across the membrane into small 4-20aa peptides that are released into the extracellular space. We find that neuroproteasome-derived extracellular peptides may be a new mechanism of neuromodulation. However, the roles for this new mechanism of neuromodulation remain completely unknown. More broadly, the functional, phenotypic, and behavioral consequences of the inhibition of neuroproteasomes remain unexplored. We have worked with chemical biologists to develop specific pharmacological tools that allow us to study this at unprecedented molecular and cellular resolution with exquisite spatiotemporal control. As we gain genetic access to this system, we seek to leverage these insights as an orthogonal means to perturb and study neuroproteasome function.
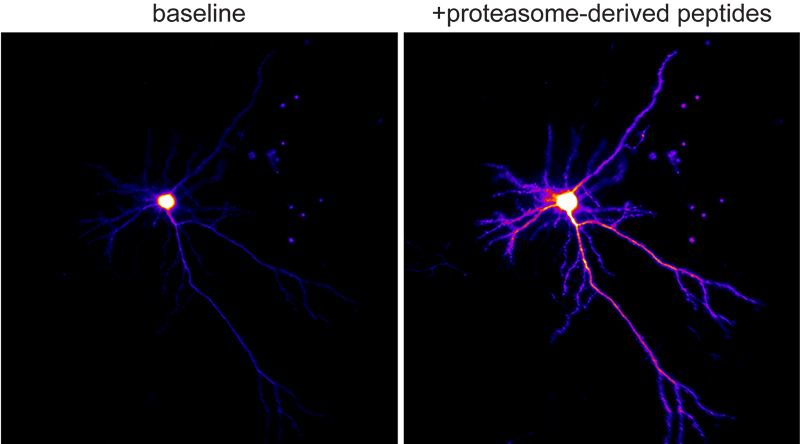
Figure 2. Ca+2 imaging of neurons responding to neuroproteasome-derived peptides.
Our broadest goals are to understand how neuroproteasomes contribute to proteostasis and to signaling in the intact mammalian brain, with the strong belief that this understanding will reveal new principles of how to think about neurodegeneration and aging. Being in the Taub Institute and being affiliated with both the Department of Neurology and the Department of Neuroscience are instrumental for realizing our goals. We use a combination of genetics, molecular manipulations, biochemistry, super-resolution and functional microscopy, and chemical biology to address these questions. We are thrilled to be at Columbia, establish collaborations across campus(es), and establish a new field in neuronal cell biology!
Kapil V. Ramachandran, PhD
Assistant Professor of Neurological Sciences (in Neurology, Neuroscience, and the Taub Institute)
kvr2113@cumc.columbia.edu

